Ann Pediatr Endocrinol Metab.
2021 Jun;26(2):118-125. 10.6065/apem.2040202.101.
Features of partial remission in children with type 1 diabetes using the insulin dose-adjusted A1c definition and risk factors associated with nonremission
- Affiliations
-
- 1Department of Paediatrics, Queen Elizabeth Hospital, Jordan, Hong Kong
- KMID: 2517590
- DOI: http://doi.org/10.6065/apem.2040202.101
Abstract
- Purpose
We sought to evaluate features of partial remission (PR) in children with type 1 diabetes mellitus (T1DM) using the insulin-dose adjusted A1c (IDAA1c) definition and to identify risk factors associated with nonremission.
Methods
Medical records of patients with newly diagnosed T1DM between January 1, 2008, and June 30, 2018, were retrospectively reviewed. Hemoglobin A1c (HbA1c) readings and insulin total daily doses (TDDs) of each patient at each follow-up visit were obtained with IDAA1c values calculated. PR was defined as an IDAA1c score of 9 points or less within 6 months of diagnosis. The trends of HbA1c and TDD within 2 years after diagnosis were compared between remitters and nonremitters. Factors that may predict the occurrence of PR were studied, with their relative risks of nonremission calculated.
Results
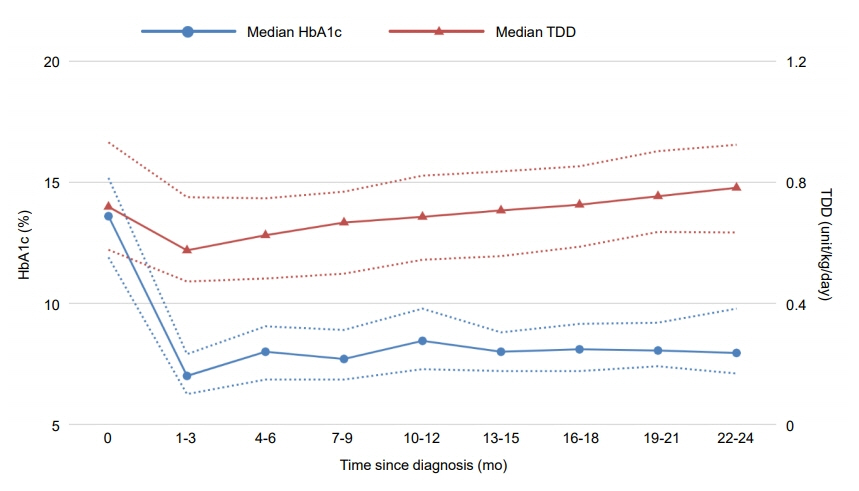
PR occurred in 26 patients (45.6%), including 8 girls and 18 boys, with a median duration of 8 months. The frequency of remission in male patients was significantly higher (P=0.002) and the relative risk of female sex with nonremission was 2.20 (95% confidence interval [CI], 1.24–3.91), which remained significant when adjusted by multivariate regression modeling. The initial HbA1c level at diagnosis was also significantly higher in the nonremission group (P=0.029), with a relative risk of 1.12 (95% CI, 1.01–1.25). Both HbA1c (P=0.012) and TDD (P=0.006) were significantly lower within 2 years after diagnosis among remitters than in nonremitters. TDD was significantly lower in male patients (P=0.029) during the same period, while there was no significant difference in HbA1c level between male and female patients (P=0.163).
Conclusion
Both the initial HbA1c level at diagnosis and sex were factors associated with the occurrence of PR. Female sex was an independent risk factor of nonremission, likely resulting from a higher insulin requirement in female T1DM patients.
Figure
Reference
-
References
1. Abdul-Rasoul M, Habib H, Al-Khouly M. "The honeymoon phase" in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006; 7:101–7.
Article2. Böber E, Dündar B, Büyükgebiz A. Partial remission phase and metabolic control in type 1 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2001; 14:435–41.
Article3. Kara Ö, Esen İ, Tepe D. Factors influencing frequency and duration of remission in children and adolescents newly diagnosed with type 1 diabetes. Med Sci Monit. 2018; 24:5996–6001.
Article4. Mortensen HB, Hougaard P, Swift P, Hansen L, Holl RW, Hoey H, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009; 32:1384–90.
Article5. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998; 128:517–23.6. Max Andersen MLC, Hougaard P, Pörksen S, Nielsen LB, Fredheim S, Svensson J, et al. Partial remission definition: validation based on the insulin dose-adjusted HbA1c (IDAA1C) in 129 Danish Children with New-Onset Type 1 Diabetes. Pediatr Diabetes. 2014; 15:469–76.
Article7. Assan R, Feutren G, Debray-Sachs M, Quiniou-Debrie MC, Laborie C, Thomas G, et al. Metabolic and immunological effects of cyclosporin in recently diagnosed type 1 diabetes mellitus. Lancet. 1985; 1:67–71.
Article8. Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. New Engl J Med. 1988; 319:599–604.
Article9. Ludvigsson J, Heding L, Lieden G, Marner B, Lernmark A. Plasmapheresis in the initial treatment of insulindependent diabetes mellitus in children. Br Med J (Clin Res Ed). 1983; 286:176–8.
Article10. Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003; 26:832–6.
Article11. Marino KR, Lundberg RL, Jasrotia A, Maranda LS, Thompson MJ, Barton BA, et al. A predictive model for lack of partial clinical remission in new-onset pediatric type 1 diabetes. PLoS One. 2017; 12:e0176860.
Article12. Nwosu BU, Zhang B, Ayyoub SS, Choi S, Villalobos-Ortiz TR, Alonso LC, et al. Children with type 1 diabetes who experienced a honeymoon phase had significantly lower LDL cholesterol 5 years after diagnosis. PLoS One. 2018; 13:e0196912.
Article13. Niedzwiecki P, Pilacinski S, Uruska A, Adamska A, Naskret D, Zozulinska-Ziolkiewicz D. Influence of remission and its duration on development of early microvascular complications in young adults with type 1 diabetes. J Diabetes Complications. 2015; 29:1105–11.
Article14. Fonolleda M, Murillo M, Vázquez F, Bel J, Vives-Pi M. Remission phase in paediatric type 1 diabetes: new understanding and emerging biomarkers. Horm Res Paediatr. 2017; 88:307–15.
Article15. Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018; 19:155–77.
Article16. Esen İ, Demirel F, Tepe D, Kara Ö. Frequency of partial remission and related factors in children and adolescents diagnosed with type 1 diabetes mellitus. Int J Diabetes Dev Ctries. 2015; 35:230–5.
Article17. Neylon OM, White M, O’Connell MA, Cameron FJ. Insulin-dose-adjusted HbA1c-defined partial remission phase in a paediatric population-when is the honeymoon over? Diabet Med. 2013; 30:627–8.18. Lundberg RL, Marino KR, Jasrotia A, Maranda LS, Barton BA, Alonso LC, et al. Partial clinical remission in type 1 diabetes: a comparison of the accuracy of total daily dose of insulin of <0.3 units/kg/day to the gold standard insulindose adjusted hemoglobin A1c of ≤9 for the detection of partial clinical remission. J Pediatr Endocrinol Metab. 2017; 30:823–30.
Article19. Pecheur A, Barrea T, Vandooren V, Beauloye V, Robert A, Lysy PA. Characteristics and determinants of partial remission in children with type 1 diabetes using the insulin-dose-adjusted A1C definition. J Diabetes Res. 2014; 2014:851378.
Article20. Nagl K, Hermann JM, Plamper M, Schröder C, Dost A, Kordonouri O, et al. Factors contributing to partial remission in type 1 diabetes: analysis based on the insulin dose-adjusted HbA1c in 3657 children and adolescents from Germany and Austria. Pediatr Diabetes. 2017; 18:428–34.
Article21. Nielens N, Pollé O, Robert A, Lysy PA. Integration of routine parameters of glycemic variability in a simple screening method for partial remission in children with type 1 diabetes. J Diabetes Res. 2018; 2018:5936360.
Article22. Mente A, Razak F, Blankenberg S, Vuksan V, Darlene Davis A, Miller R, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetic Care. 2010; 33:1629–34.
Article23. Ehtisham S, Crabtree N, Clark P, Shaw N, Barrett T. Ethnic differences in insulin resistance and body composition in United Kingdom adolescents. J Clin Endocrinol Metab. 2005; 90:3963–9.
Article24. Chase HP, MacKenzie TA, Burdick J, Fiallo-Scharer R, Walravens P, Klingensmith G, et al. Redefining the clinical remission period in children with type 1 diabetes. Pediatr Diabetes. 2004; 5:16–9.
Article25. Aly H, Gottlieb P. The honeymoon phase: intersection of metabolism and immunology. Curr Opin Endocrinol Diabetes Obes. 2009; 16:286–92.
Article26. Gleason CE, Gonzalez M, Harmon JS, Paul Robertson R. Determinants of glucose toxicity and its reversibility in the pancreatic islet β-cell line, HIT-T15. Am J Physiol Endocrinol Metab. 2000; 279:E997–1002.
Article27. Dost A, Herbst A, Kintzel K, Haberland H, Roth CL, Gortner L, et al. Shorter remission period in young versus older children with diabetes mellitus type 1. Exp Clin Endocrinol Diabetes. 2007; 115:33–7.
Article28. von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007; 7:988–94.
Article29. Turtinen M, Härkönen T, Parkkola A, Ilonen J, Knip M. Sex as a determinant of type 1 diabetes at diagnosis. Pediatr Diabetes. 2018; 19:1221–8.
Article30. Crinò A, Pediatrico O, Gesù B, Gross TM, Pozzilli P, Mesturino CA, et al. Is the process of β-cell destruction in type 1 diabetes at time of diagnosis more extensive in females than in males? Is the process of b-cell destruction in type 1 diabetes at time of diagnosis more extensive in females than in males? Eur J Endocrinol. 2001; 145:757–61.31. Arslanian SA, Heil BV, Becker DJ, Drash AL. Sexual dimorphism in insulin sensitivity in adolescents with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991; 72:920–6.
Article32. Komulainen J, Åkerblom HK, Lounamaa R, Knip M. Prepubertal girls with insulin-dependent diabetes mellitus have higher exogenous insulin requirement than boys. Eur J Pediatr. 1998; 157:708–11.
Article33. Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes. 2008; 9(3 Pt 1):197–201.
Article34. Szypowska A, Skórka A. The risk factors of ketoacidosis in children with newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes. 2011; 12(4 Pt 1):302–6.
Article35. VanBuecken DE, Greenbaum CJ. Residual C-peptide in type 1 diabetes: what do we really know? Pediatr Diabetes. 2014; 15:84–90.
Article36. Ludvigsson J, Carlsson A, Deli A, Forsander G, Ivarsson SA, Kockum I, et al. Decline of C-peptide during the first year after diagnosis of type 1 diabetes in children and adolescents. Diabetes Res Clin Pract. 2013; 100:203–9.
Article37. Pyziak A, Zmyslowska A, Bobeff K, Malachowska B, Fendler W, Wyka K, et al. Markers influencing the presence of partial clinical remission in patients with newly diagnosed type 1 diabetes. J Pediatr Endocrinol Metab. 2017; 30:1147–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Successful Type 2 Diabetes Control with Lifestyle Modification in Children and Adolescents
- Effective Use of Insulin Pump in Patients with Type 1 Diabetes
- Factors Influencing the Onset of Honeymoon Period in Children with Type I Diabetes Mellitus
- Insulin Deficiency is an Important Risk Factor for the Development of Type 2 Diabetes in Asia
- Type 2 diabetes mellitus and metabolic syndrome