Korean J Gastroenterol.
2021 Jun;77(6):309-312. 10.4166/kjg.2021.035.
Russell Body Lesions of the Colon: Case Report and Literature Review
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Hinchingbrooke Hospital, North West Anglia NHS Foundation Trust, Huntingdon, United Kingdom
- 2MRC Cancer Unit, University of Cambridge, Cambridge, United Kingdom
- 3Department of Gastroenterology, Addenbrookes Hospital, Cambridge University NHS Foundation Trust, Cambridge, United Kingdom
- KMID: 2516893
- DOI: http://doi.org/10.4166/kjg.2021.035
Abstract
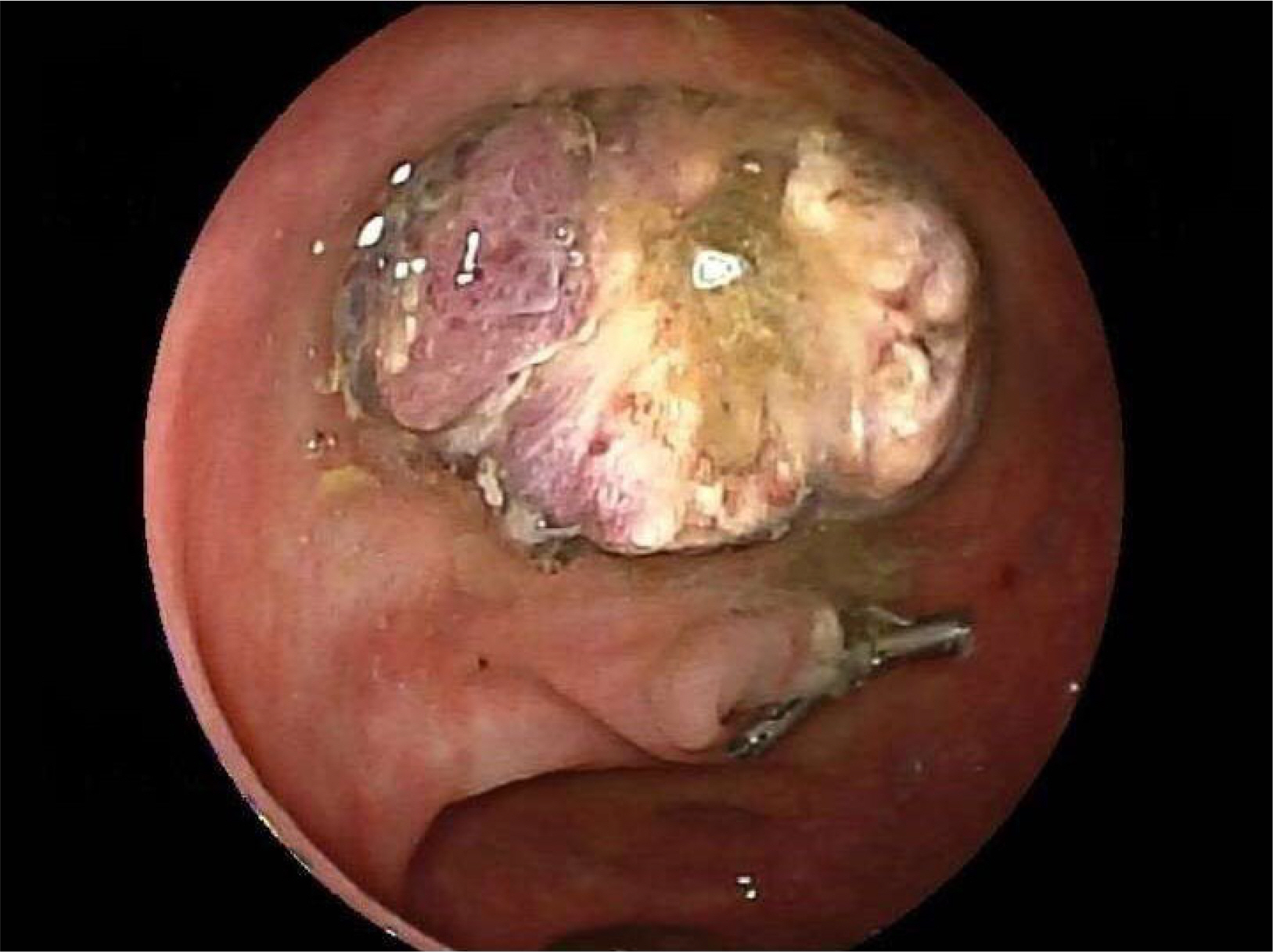
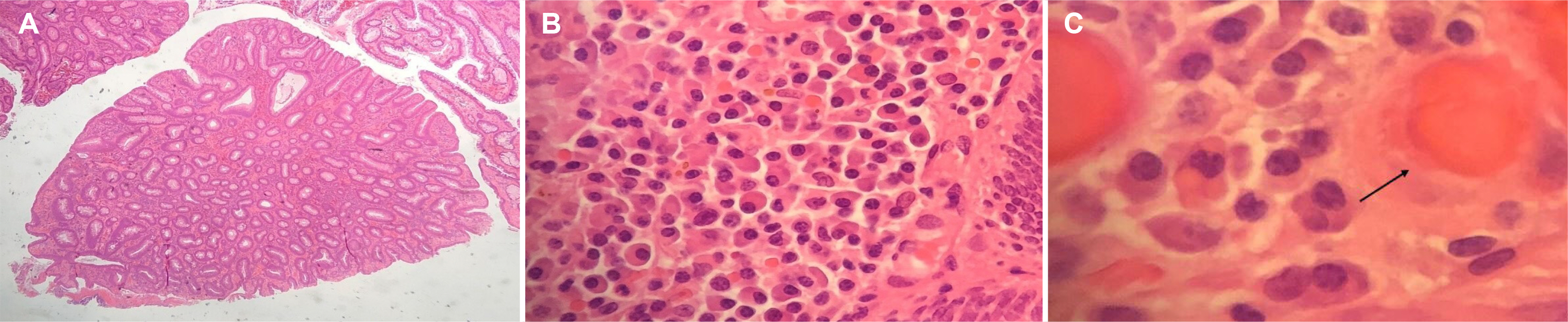
- Russell bodies (RB) are rare manifestations within the lower gastrointestinal tract. To date, there are only three other reported cases of RB lesions of the colon; two were polyps, and the third was a case of a multifocal RB lesion of the gastrointestinal tract. This paper reports a case of a tubulovillous adenoma with RB of the sigmoid colon in a patient diagnosed incidentally as part of the UK National Health Service Bowel Cancer Screening Programme. A thorough hematological investigation is required to exclude hematological malignancies because of its association with plasma cell neoplasm. These lesions should undergo clonality analysis to exclude the monoclonal proliferation of plasma cells. Ideally, a bone marrow aspirate and investigations for amyloidosis should be performed to exclude underlying hematological malignancies.
Keyword
Figure
Reference
-
1. Mott FW. 1905; Observations on the brains of men and animals infected with various forms of trypanosomes. Preliminary note. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. 76:235–242. DOI: 10.1098/rspb.1905.0016.2. Reinhard H, Karamchandani DM. 2018; Russell bodies and russell body inflammatory polyp in the colorectum: a review of clinicopathologic features. Biomed Res Int. 2018:2845291. DOI: 10.1155/2018/2845291. PMID: 30151376. PMCID: PMC6087615.
Article3. Brink T, Wagner B, Gebbers JO. 1999; Monoclonal plasma and Mott cells in a rectal adenoma. Histopathology. 34:81–82. DOI: 10.1046/j.1365-2559.1999.00550.x. PMID: 9934591.
Article4. Coates RF, Ferrentino N, Yang MX. 2017; Russell body inflammatory polyp. Int J Surg Pathol. 25:94–96. DOI: 10.1177/1066896916666318. PMID: 27571792.
Article5. Muthukumarana V, Segura S, O'Brien M, Siddiqui R, El-Fanek H. 2015; “Russell body gastroenterocolitis” in a posttransplant patient: a case report and review of literature. Int J Surg Pathol. 23:667–672. DOI: 10.1177/1066896915601893. PMID: 26310272.
Article6. Russell W. 1890; An address on a characteristic organism of cancer. Br Med J. 2:1356–1360. DOI: 10.1136/bmj.2.1563.1356. PMID: 20753194. PMCID: PMC2208600.
Article7. Bain BJ. 2009; Russell bodies and Mott cells. Am J Hematol. 84:516. DOI: 10.1002/ajh.21397. PMID: 19384941.
Article8. Tazawa K, Tsutsumi Y. 1998; Localized accumulation of Russell body-containing plasma cells in gastric mucosa with Helicobacter pylori infection: 'Russell body gastritis'. Pathol Int. 48:242–244. DOI: 10.1111/j.1440-1827.1998.tb03901.x. PMID: 9589496.9. Martín-Noya A, Ríos-Herranz E, Rafel-Ribas E. 1999; Multiple myeloma with numerous intranuclear Russell bodies. Haematologica. 84:179–180. PMID: 10091418.10. van Gammeren AJ, Alcala LS, Smolders M, Boersma RS. 2015; Numerous Russell bodies and Dutcher bodies in multiple myeloma. Br J Haematol. 170:743. DOI: 10.1111/bjh.13181. PMID: 25312850.
Article11. Sachchithanantham S, Wechalekar AD. 2013; Imaging in systemic amyloidosis. Br Med Bull. 107:41–56. DOI: 10.1093/bmb/ldt021. PMID: 23896486.
Article12. Hazenberg BP, van Rijswijk MH, Piers DA, et al. 2006; Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. Am J Med. 119:355.e15–355.e3.55E24. DOI: 10.1016/j.amjmed.2005.08.043. PMID: 16564782.
Article13. Wisniowski B, Wechalekar A. 2020; Confirming the diagnosis of amyloidosis. Acta Haematol. 143:312–321. DOI: 10.1159/000508022. PMID: 32544917.
Article14. Rutter MD, East J, Rees CJ, et al. 2020; British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 69:201–223. DOI: 10.1136/gutjnl-2019-319858. PMID: 31776230. PMCID: PMC6984062.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Russell Body Gastritis Associated with Helicobacter pylori Infection
- Two Cases of Russell Body Gastritis Treated by Helicobacter pylori Eradication
- Russell Body Gastritis Disappeared after Helicobacter pylori Eradication
- A Case Report of Familial Silver-Russell Syndrome
- Regression of Russell Body Gastritis after Helicobacter pylori Eradication