Diabetes Metab J.
2021 May;45(3):349-357. 10.4093/dmj.2019.0211.
Association of Urinary N-Acetyl-β-D-Glucosaminidase with Cardiovascular Autonomic Neuropathy in Type 1 Diabetes Mellitus without Nephropathy
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Medicine, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
- 5Department of Clinical Research Design & Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Korea
- KMID: 2516337
- DOI: http://doi.org/10.4093/dmj.2019.0211
Abstract
- Background
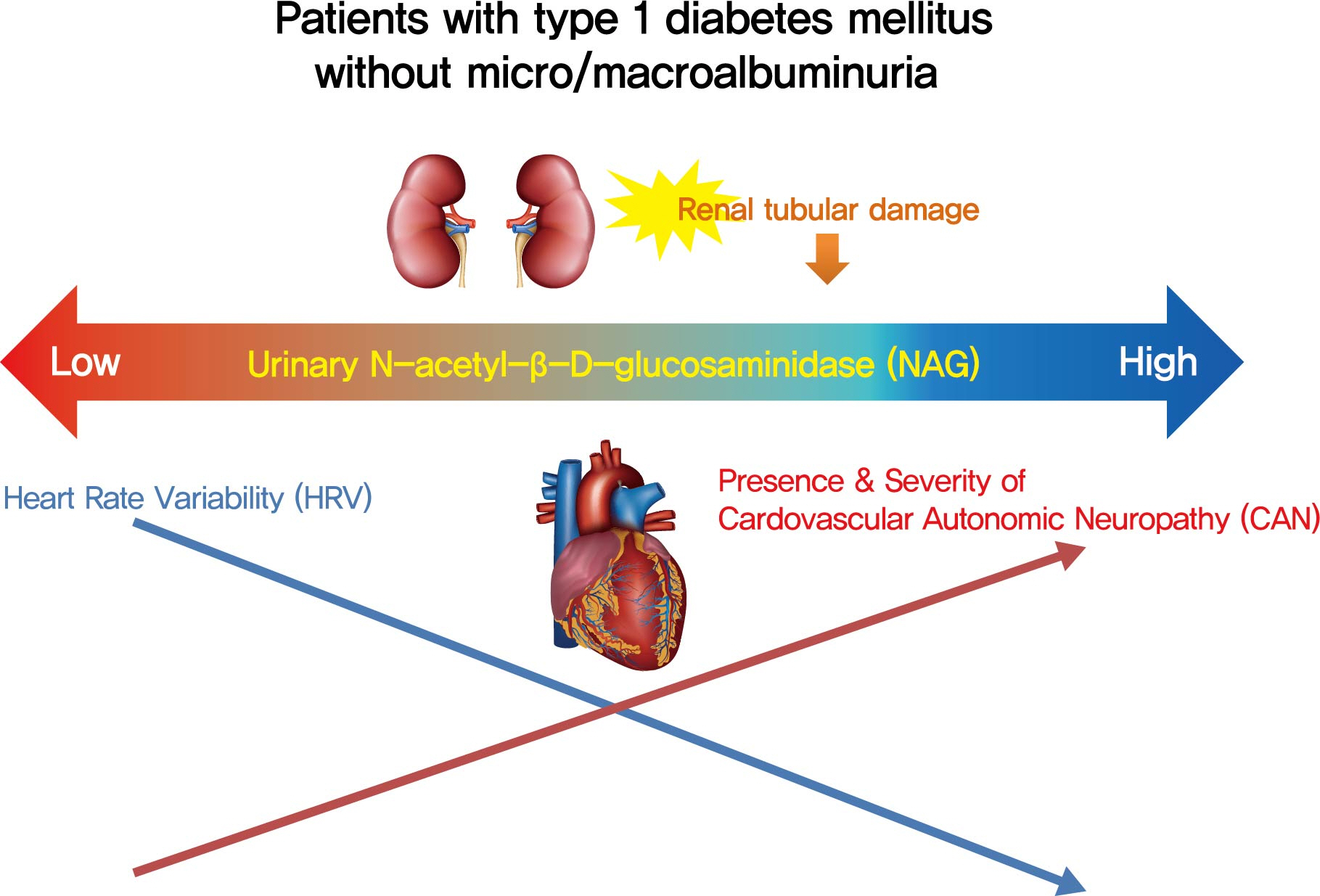
Cardiovascular autonomic neuropathy (CAN) is a common microvascular complication of diabetes and related to albuminuria in diabetic nephropathy (DN). Urinary N-acetyl-β-D-glucosaminidase (uNAG) is a renal tubular injury marker which has been reported as an early marker of DN even in patients with normoalbuminuria. This study evaluated whether uNAG is associated with the presence and severity of CAN in patients with type 1 diabetes mellitus (T1DM) without nephropathy.
Methods
This cross-sectional study comprised 247 subjects with T1DM without chronic kidney disease and albuminuria who had results for both uNAG and autonomic function tests within 3 months. The presence of CAN was assessed by age-dependent reference values for four autonomic function tests. Total CAN score was assessed as the sum of the partial points of five cardiovascular reflex tests and was used to estimatethe severity of CAN. The correlations between uNAG and heart rate variability (HRV) parameters were analyzed.
Results
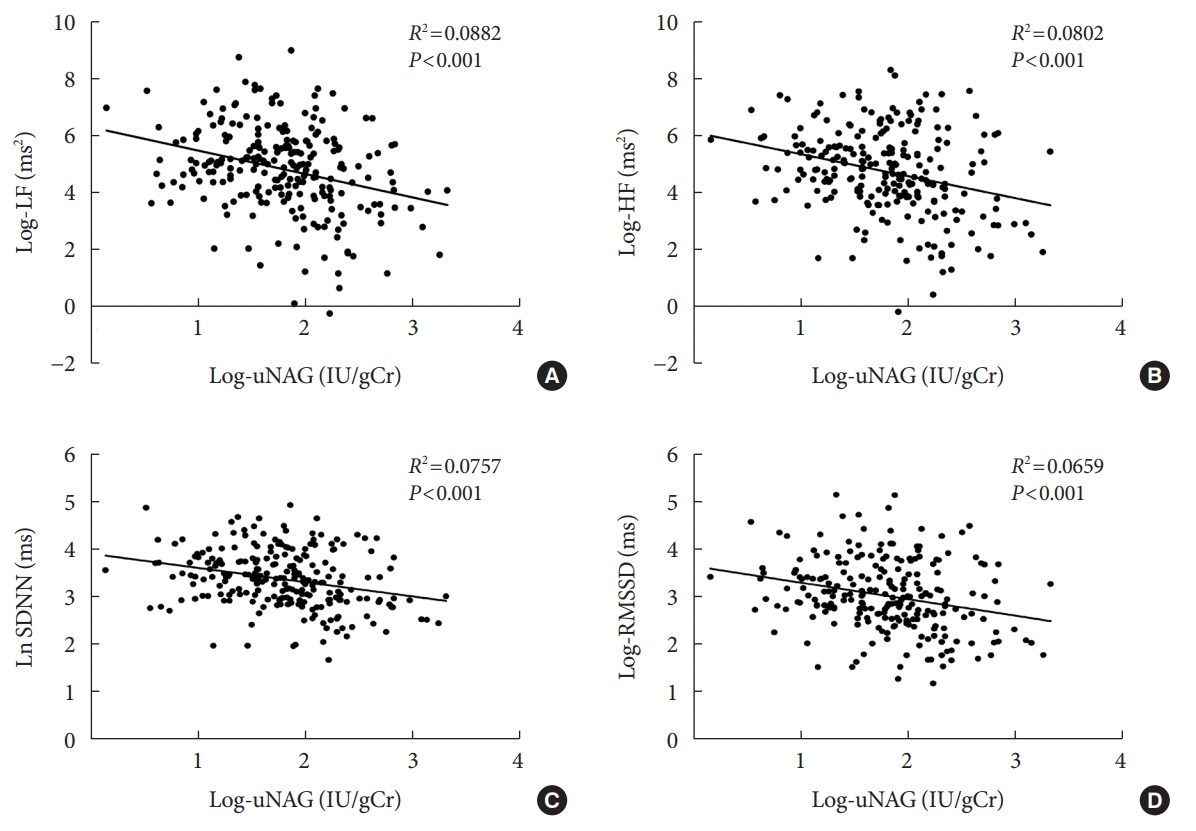
The association between log-uNAG and presence of CAN was significant in a multivariate logistic regression model (adjusted odds ratio, 2.39; 95% confidence interval [CI], 1.08 to 5.28; P=0.031). Total CAN score was positively associated with loguNAG (β=0.261, P=0.026) in the multivariate linear regression model. Log-uNAG was inversely correlated with frequency-domain and time-domain indices of HRV.
Conclusion
This study verified the association of uNAG with presence and severity of CAN and changes in HRV in T1DM patients without nephropathy. The potential role of uNAG should be further assessed for high-risk patients for CAN in T1DM patients without nephropathy.
Figure
Reference
-
1. Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. Nephropathy in diabetes. Diabetes Care. 2004; 27 Suppl 1:S79–83.2. Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. 2012; 32:452–62.
Article3. Fu WJ, Xiong SL, Fang YG, Wen S, Chen ML, Deng RT, et al. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine. 2012; 41:82–8.
Article4. Hong CY, Chia KS. Markers of diabetic nephropathy. J Diabetes Complications. 1998; 12:43–60.
Article5. Omozee EB, Okaka EI, Edo AE, Obika LF. Urinary N-acetyl-beta-d-glucosaminidase levels in diabetic adults. J Lab Physicians. 2019; 11:1–4.
Article6. Nauta FL, Boertien WE, Bakker SJ, van Goor H, van Oeveren W, de Jong PE, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care. 2011; 34:975–81.
Article7. Schumer MP, Joyner SA, Pfeifer MA. Cardiovascular autonomic neuropathy testing in patients with diabetes. Diabetes Spectr. 1998; 11:227–31.8. Krentz AJ, Clough G, Byrne CD. Interactions between microvascular and macrovascular disease in diabetes: pathophysiology and therapeutic implications. Diabetes Obes Metab. 2007; 9:781–91.
Article9. Said SM, Nasr SH. Silent diabetic nephropathy. Kidney Int. 2016; 90:24–6.
Article10. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001; 286:421–6.
Article11. Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011; 27:639–53.
Article12. Jin SM, Baek JH, Suh S, Jung CH, Lee WJ, Park CY, et al. Factors associated with greater benefit of a national reimbursement policy for blood glucose test strips in adult patients with type 1 diabetes: a prospective cohort study. J Diabetes Investig. 2017; 9:549–57.
Article13. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014; 63:713–35.
Article14. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014; 37:2864–83.
Article15. Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985; 8:491–8.
Article16. O’Brien IA, O’Hare JP, Lewin IG, Corrall RJ. The prevalence of autonomic neuropathy in insulin-dependent diabetes mellitus: a controlled study based on heart rate variability. Q J Med. 1986; 61:957–67.17. Jun JE, Jin SM, Baek J, Oh S, Hur KY, Lee MS, et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015; 14:70.
Article18. Lorini R, Scaramuzza A, Cortona L, Valenti G, d’Annunzio G, Melzi d’Eril GV. Increased urinary N-acetyl-beta-glucosaminidase (NAG) excretion in young insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1995; 29:99–105.19. Lee M, Hong N, Lee YH, Kang ES, Cha BS, Lee BW. Elevated N-acetyl-β-d-glucosaminidase, a urinary tubular damage marker, is a significant predictor of carotid artery atherosclerosis in type 1 diabetes, independent of albuminuria: a cross-sectional study. J Diabetes Complications. 2018; 32:777–83.20. Kopf S, Oikonomou D, Zdunek D, von Eynatten M, Hess G, Nawroth PP, et al. Urinary N-acetyl-beta-d-glucosaminidase excretion: an indicator of neuropathy in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2013; 121:601–6.
Article21. Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes. 2002; 51:3524–31.
Article22. Cha SA, Park YM, Yun JS, Lee SH, Ahn YB, Kim SR, et al. Time- and frequency-domain measures of heart rate variability predict cardiovascular outcome in patients with type 2 diabetes. Diabetes Res Clin Pract. 2018; 143:159–69.
Article23. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017; 5:258.
Article24. Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019; 43:3–30.
Article25. Orlov S, Cherney DZ, Pop-Busui R, Lovblom LE, Ficociello LH, Smiles AM, et al. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin J Am Soc Nephrol. 2015; 10:1136–44.
Article26. Sundkvist G, Lilja B. Autonomic neuropathy predicts deterioration in glomerular filtration rate in patients with IDDM. Diabetes Care. 1993; 16:773–9.
Article27. Spallone V, Maiello MR, Kurukulasuriya N, Barini A, Lovecchio M, Tartaglione R, et al. Does autonomic neuropathy play a role in erythropoietin regulation in non-proteinuric type 2 diabetic patients? Diabet Med. 2004; 21:1174–80.
Article28. Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008; 4:216–26.
Article29. Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994; 10:339–83.
Article30. Meinhold JA, Maslowska-Wessel E, Bender R, Sawicki PT. Low prevalence of cardiac autonomic neuropathy in type 1 diabetic patients without nephropathy. Diabet Med. 2001; 18:607–13.
Article31. Chen HT, Lin HD, Won JG, Lee CH, Wu SC, Lin JD, et al. Cardiovascular autonomic neuropathy, autonomic symptoms and diabetic complications in 674 type 2 diabetes. Diabetes Res Clin Pract. 2008; 82:282–90.
Article32. Jun JE, Lee SE, Lee YB, Ahn JY, Kim G, Hur KY, et al. Continuous glucose monitoring defined glucose variability is associated with cardiovascular autonomic neuropathy in type 1 diabetes. Diabetes Metab Res Rev. 2019; 35:e3092.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between Cardiovascular Autonomic Neuropathy and Diabetic Retinopathy in Patients with Non-Insulin Dependent Diabetes Mellitus
- Relationship between Peripheral Neuropathy and Cardiovascular Autonomic Neuropathy in Non-Insulin Dependent Diabetics
- The Difference of Autonomic Dysfunction according to the Absence or Presence of Nephropathy in NIDDM Patients
- Relationship between autonomic and peripheral neuropathies and cardiovascular outcomes in diabetes
- Low tear production in patients with Diabetes Mellitus