Clin Endosc.
2021 May;54(3):420-427. 10.5946/ce.2020.184.
Confirming Whether Fine Needle Biopsy Device Shortens the Learning Curve of Endoscopic Ultrasound-Guided Tissue Acquisition Without Rapid Onsite Evaluation
- Affiliations
-
- 1Department of Internal Medicine, National Cheng Kung University Hospital, Tainan
- 2Department of pathology, National Cheng Kung University Hospital, Tainan, Taiwan
- 3Department of Gastroenterology, Kitasato University School of Medicine, Kanagawa, Japan
- 4Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- KMID: 2516323
- DOI: http://doi.org/10.5946/ce.2020.184
Abstract
- Background/Aims
Endoscopic ultrasonography (EUS)-guided tissue acquisition requires a long learning curve. We aimed to compare the skill maturation curves between fine needle aspiration (FNA) and biopsy (FNB) for tissue acquisition.
Methods
The initial 60 procedures performed by the trainee endosonographer (30 FNA vs. 30 FNB) were consecutively enrolled. The difference in procedure performance was compared between the two groups. Learning curves were assessed. Twenty additional cases were subsequently enrolled to assess the consistency of performance in the FNB group.
Results
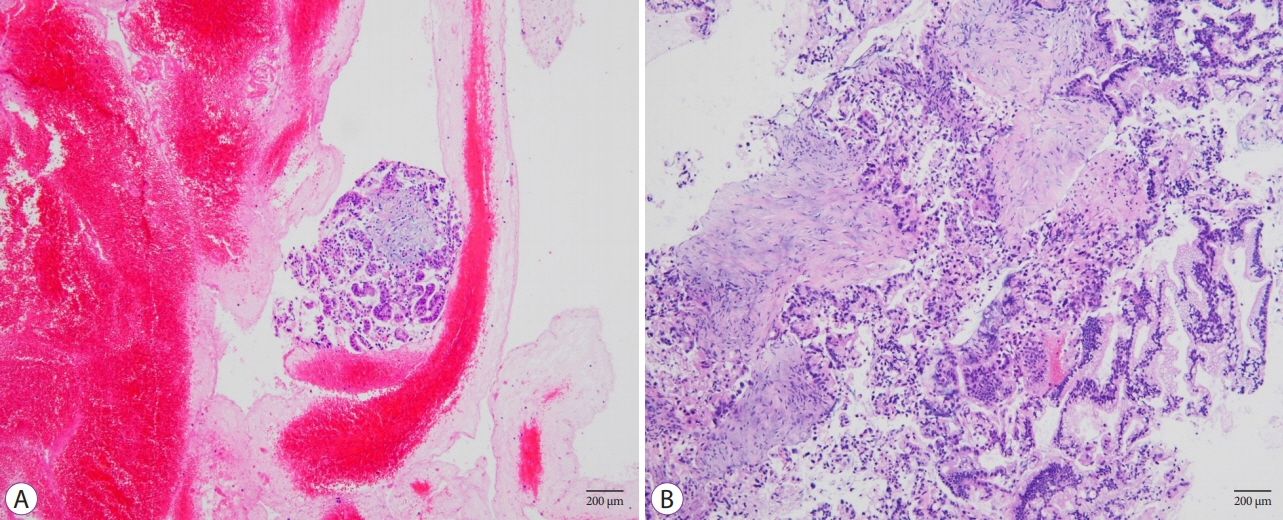
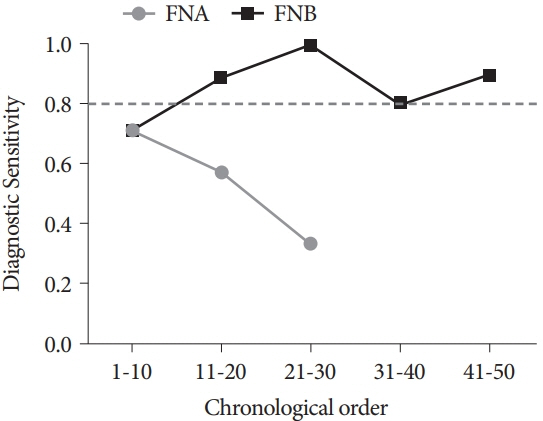
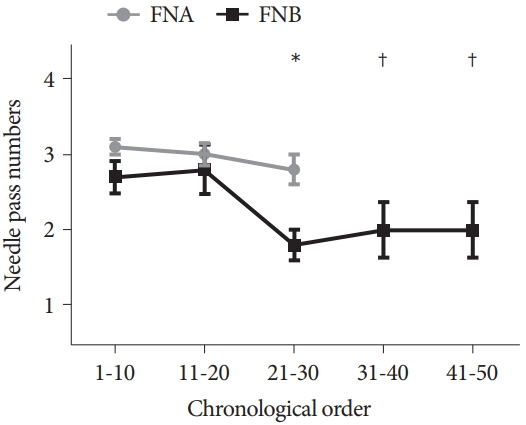
The FNB group acquired larger tissue samples (2.35 vs. 0.70 mm2; p<0.001) with lower blood content (p=0.001) and higher tissue quality (p=0.017) compared with the FNA group. In addition, the FNB group required less needle pass to establish a diagnosis (2.43 vs. 2.97; p=0.006). A threshold diagnostic sensitivity of ≥80% was achieved after performing 10 FNB procedures. The number of needle passes significantly decreased after conducting 20 FNB procedures (1.80 vs. 2.70; p=0.041). The diagnostic sensitivity and number of needle passes remained the same in the subsequent FNB procedures. By contrast, this skill maturation phenomenon was not observed after performing 30 FNA procedures.
Conclusions
In EUS-guided tissue acquisition, the FNB needle was more efficient and thus shortened the learning curve of EUSguided tissue acquisition in trainee endosonographers.
Keyword
Figure
Reference
-
1. Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992; 38:172–173.
Article2. Mohammad Alizadeh AH, Shahrokh S, Hadizadeh M, Padashi M, Zali MR. Diagnostic potency of EUS-guided FNA for the evaluation of pancreatic mass lesions. Endosc Ultrasound. 2016; 5:30–34.
Article3. Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006; 63:966–975.
Article4. Okasha HH, Naga MI, Esmat S, et al. Endoscopic ultrasound-guided fine needle aspiration versus percutaneous ultrasound-guided fine needle aspiration in diagnosis of focal pancreatic masses. Endosc Ultrasound. 2013; 2:190–193.
Article5. Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000; 51:184–190.
Article6. Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol. 2000; 95:2248–2254.
Article7. Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: a single-center experience. Dig Endosc. 2011; 23 Suppl 1:34–38.
Article8. Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001; 54:811–814.
Article9. Wani S, Coté GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc. 2013; 77:558–565.
Article10. Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012; 44:190–206.
Article11. Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004; 59:33–37.
Article12. Cho IR, Chung MJ, Bang S, et al. Gemcitabine based neoadjuvant chemoradiotherapy therapy in patients with borderline resectable pancreatic cancer. Pancreatology. 2013; 13:539–543.
Article13. Petrelli F, Coinu A, Borgonovo K, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015; 44:515–521.14. Naveed M, Siddiqui AA, Kowalski TE, et al. A Multicenter comparative trial of a novel EUS-guided core biopsy needle (SharkCoreTM) with the 22-gauge needle in patients with solid pancreatic mass lesions. Endosc Ultrasound. 2018; 7:34–40.15. Haseeb A, Taylor LJ, Adler DG. Comparing endoscopic ultrasound-guided core biopsies of solid pancreatic and extrapancreatic lesions: a large single-operator experience with a new fine-needle biopsy needle. Ann Gastroenterol. 2018; 31:742–746.
Article16. Witt BL, Factor RE, Chadwick BE, Caron J, Siddiqui AA, Adler DG. Evaluation of the SharkCore® needle for EUS-guided core biopsy of pancreatic neuroendocrine tumors. Endosc Ultrasound. 2018; 7:323–328.
Article17. Meenan J, Harris K, Oppong K, et al. Service provision and training for endoscopic ultrasound in the UK. Frontline Gastroenterol. 2011; 2:188–194.
Article18. Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, Vilmann P, Bhutani MS. A quarter century of EUS-FNA: progress, milestones, and future directions. Endosc Ultrasound. 2018; 7:141–160.
Article19. Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study). Gastrointest Endosc. 2015; 81:177–185.
Article20. Ishiwatari H, Sato J, Fujie S, et al. Gross visual inspection by endosonographers during endoscopic ultrasound-guided fine needle aspiration. Pancreatology. 2019; 19:191–195.
Article21. Leung Ki E-L, Lemaistre A-I, Fumex F, et al. Macroscopic onsite evaluation using endoscopic ultrasound fine needle biopsy as an alternative to rapid onsite evaluation. Endosc Int Open. 2019; 7:E189–E194.
Article22. Oh D, Seo DW, Hong SM, et al. The impact of macroscopic on-site evaluation using filter paper in EUS-guided fine-needle biopsy. Endosc Ultrasound. 2019; 8:342–347.
Article23. Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003; 58:690–695.
Article24. Harewood GC, Wiersema LM, Halling AC, Keeney GL, Salamao DR, Wiersema MJ. Influence of EUS training and pathology interpretation on accuracy of EUS-guided fine needle aspiration of pancreatic masses. Gastrointest Endosc. 2002; 55:669–673.
Article25. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997; 112:1087–1095.
Article26. DiMaio CJ, Kolb JM, Benias PC, et al. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): results of a large North American multicenter study. Endosc Int Open. 2016; 4:E974–E979.
Article27. Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: a case-control study. Gastrointest Endosc. 2016; 84:1034–1039.
Article28. Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018; 67:2081–2084.
Article29. Mukai S, Itoi T, Yamaguchi H, et al. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019; 8:50–57.
Article30. Wang J, Zhao S, Chen Y, Jia R, Zhang X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: A meta-analysis. Medicine (Baltimore). 2017; 96:e7452.31. Altonbary A, Hakim H, Bakr D, El-Shamy A, Elkashef W. Comparison of endoscopic ultrasound-guided tissue acquisition using 22 G versus 20 G procore needles in solid lesions: a pilot study. Egypt J Intern Med. 2019; 31:266–272.
Article32. Affolter KE, Schmidt RL, Matynia AP, Adler DG, Factor RE. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: a systematic review and meta-analysis. Dig Dis Sci. 2013; 58:1026–1034.
Article33. Ramesh J, Bang JY, Hebert-Magee S, et al. Randomized Trial Comparing the Flexible 19G and 25G Needles for Endoscopic Ultrasound-Guided Fine Needle Aspiration of Solid Pancreatic Mass Lesions. Pancreas. 2015; 44:128–133.
Article34. Facciorusso A, Wani S, Triantafyllou K, et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc. 2019; 90:893–903.e7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors
- Procore and Flexible 19 Gauge Needle Can Replace Trucut Biopsy Needle?
- Present and Future of Endoscopic Ultrasound-Guided Tissue Acquisition in Solid Pancreatic Tumors
- Prospective Assessment of the Performance of a New Fine Needle Biopsy Device for EUS-Guided Sampling of Solid Lesions