Prospective Assessment of the Performance of a New Fine Needle Biopsy Device for EUS-Guided Sampling of Solid Lesions
- Affiliations
-
- 1Division of Gastroenterology, Section of Interventional Endoscopy, Indiana University School of Medicine, Indianapolis, IN, USA. moalhadd@iu.edu
- 2Department of Laboratory Medicine and Cytopathology, Indiana University School of Medicine, Indianapolis, IN, USA.
- KMID: 2430401
- DOI: http://doi.org/10.5946/ce.2018.053
Abstract
- BACKGROUND/AIMS
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) remains the most common EUS-guided tissue acquisition technique. This study aimed to evaluate the performance of a new Franseen tip fine needle biopsy (FNB) device for EUSguided sampling of solid lesions and compare it with the historical FNA technique.
METHODS
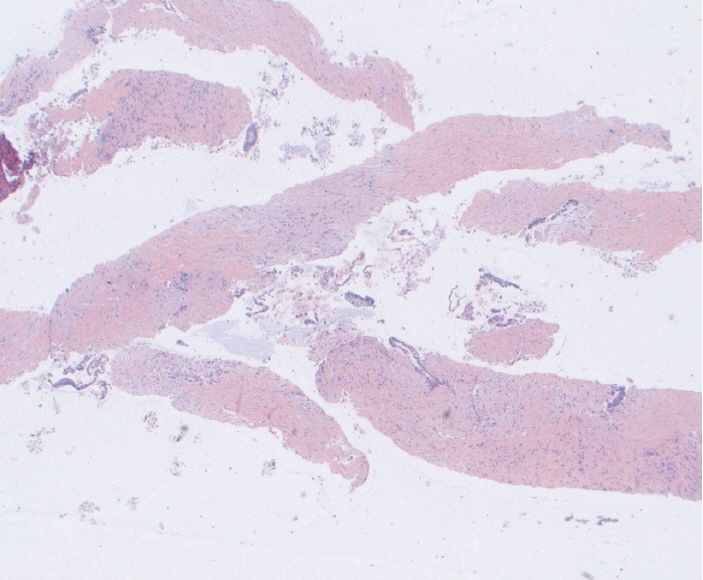
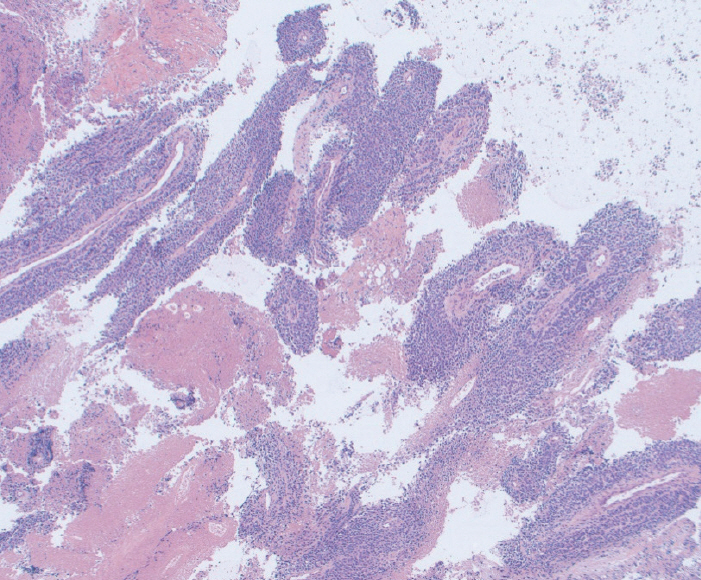
Acquire® 22 G FNB needle (Boston Scientific Co., Natick, MA, USA) was used for solid tumor sampling (Study group). Tissue was collected for rapid on-site evaluation, and touch and crush preparations were made. Historical EUS-FNA samples obtained using Expect® 22 G FNA needle (Boston Scientific Co.) were used as controls (Control group). All specimens were independently evaluated by two cytopathologists blinded to the formal cytopathological diagnosis.
RESULTS
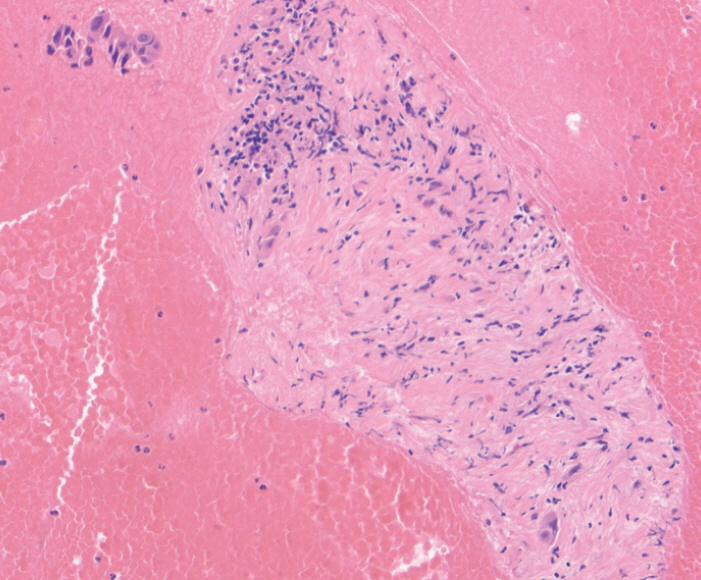
Mean cell block histology scores were significantly higher (p=0.046) in the FNB group (51 samples) despite a significantly lower (p < 0.001) mean number of passes compared to the FNA group (50 specimens). The overall diagnostic yields for the FNB vs. FNA groups were 96% vs. 88%. The degree of tumor differentiation was adequately assessed in all cell block qualifying lesions in the FNB group. Two patients developed post-FNB abdominal pain.
CONCLUSIONS
The new Franseen tip FNB device provides histologically superior and cytologically comparable specimens to those obtained by FNA, but with fewer passes.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Endoscopic Ultrasound Fine-Needle Aspiration versus Fine-Needle Biopsy for Lymph Node Diagnosis: A Large Multicenter Comparative Analysis
Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Pichamol Jirapinyo, Igor Braga Ribeiro, Galileu Ferreira Ayala Farias, Marvin Ryou, Linda S. Lee, Christopher C. Thompson
Clin Endosc. 2020;53(5):600-610. doi: 10.5946/ce.2019.170.Endoscopic Ultrasound-Guided Liver Biopsies: Is the Future Here Yet?
Ihab I. El Hajj, Mohammad Al-Haddad
Clin Endosc. 2019;52(4):297-298. doi: 10.5946/ce.2019.126.Present and Future of Endoscopic Ultrasound-Guided Tissue Acquisition in Solid Pancreatic Tumors
Jae Keun Park, Kwang Hyuck Lee
Clin Endosc. 2019;52(6):541-548. doi: 10.5946/ce.2019.127.Will New Instruments for Endoscopic Ultrasound-Guided Tissue Acquisition Make Us Happy?
Chang-Il Kwon
Clin Endosc. 2018;51(6):510-512. doi: 10.5946/ce.2018.164.
Reference
-
1. Wani S, Muthusamy VR, Komanduri S, et al. EUS-guided tissue acquisition: an evidence-based approach (with videos). Gastrointest Endosc. 2014; 80:939–959.e7.
Article2. Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline - updated January 2017. Endoscopy. 2017; 49:695–714.
Article3. Mohamadnejad M, Mullady D, Early DS, et al. Increasing number of passes beyond 4 does not increase sensitivity of detection of pancreatic malignancy by endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2017; 15:1071–1078.e2.
Article4. Rong L, Kida M, Yamauchi H, et al. Factors affecting the diagnostic accuracy of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Dig Endosc. 2012; 24:358–363.
Article5. Shield PW, Cosier J, Ellerby G, Gartrell M, Papadimos D. Rapid on-site evaluation of fine needle aspiration specimens by cytology scientists: a review of 3032 specimens. Cytopathology. 2014; 25:322–329.
Article6. Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015; 110:1429–1439.
Article7. Savides TJ, Donohue M, Hunt G, et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: a benchmark for quality performance measurement. Gastrointest Endosc. 2007; 66:277–282.8. Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009; 70:254–261.
Article9. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919.
Article10. Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004; 36:397–401.
Article11. Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003; 57:101–106.
Article12. DeWitt J, Cho CM, Lin J, et al. Comparison of EUS-guided tissue acquisition using two different 19-gauge core biopsy needles: a multicenter, prospective, randomized, and blinded study. Endosc Int Open. 2015; 3:E471–E478.
Article13. Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016; 48:339–349.
Article14. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
Article15. Bang JY, Varadarajulu S. Procore and flexible 19 gauge needle can replace trucut biopsy needle? Clin Endosc. 2013; 46:503–505.
Article16. Witt BL, Adler DG, Hilden K, Layfield LJ. A comparative needle study: EUS-FNA procedures using the HD ProCore™ and EchoTip® 22-gauge needle types. Diagn Cytopathol. 2013; 41:1069–1074.17. Nayar MK, Paranandi B, Dawwas MF, et al. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest Endosc. 2017; 85:1017–1024.
Article18. Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: a case-control study. Gastrointest Endosc. 2016; 84:1034–1039.
Article19. Rodrigues-Pinto E, Jalaj S, Grimm IS, Baron TH. Impact of EUS-guided fine-needle biopsy sampling with a new core needle on the need for onsite cytopathologic assessment: a preliminary study. Gastrointest Endosc. 2016; 84:1040–1046.
Article20. Bang JY, Hebert-Magee S, Hasan MK, Navaneethan U, Hawes R, Varadarajulu S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: initial assessment. Dig Endosc. 2017; 29:338–346.
Article21. Mounzer R, Yen R, Marshall C, et al. Interobserver agreement among cytopathologists in the evaluation of pancreatic endoscopic ultrasound-guided fine needle aspiration cytology specimens. Endosc Int Open. 2016; 4:E812–E819.
Article22. Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012; 75:319–331.
Article23. Aadam AA, Wani S, Amick A, et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016; 4:E497–E505.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- Addition of Endoscopic Ultrasound (EUS)-Guided Fine Needle Aspiration and On-Site Cytology to EUS-Guided Fine Needle Biopsy Increases Procedure Time but Not Diagnostic Accuracy
- How to optimize the diagnostic yield of endoscopic ultrasoundguided fine-needle sampling in solid pancreatic lesions from a technical perspective
- Review of the 2017 European Society of Gastrointestinal Endoscopy Guidelines for Endoscopic Ultrasound - Guided Sampling in Pancreaticobiliary Lesions
- Present and Future of Endoscopic Ultrasound-Guided Tissue Acquisition in Solid Pancreatic Tumors