Int J Stem Cells.
2021 May;14(2):203-211. 10.15283/ijsc21015.
Distinct Repopulation Activity in Hu-Mice between CBand LPB-CD34+ Cells by Enrichment of Transcription Factors
- Affiliations
-
- 1Department of Biomedical Science, CHA University, Seongnam, Korea
- 2CHA Advanced Research Institute, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 3Department of Radiation Oncology, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- KMID: 2515996
- DOI: http://doi.org/10.15283/ijsc21015
Abstract
- Background and Objectives
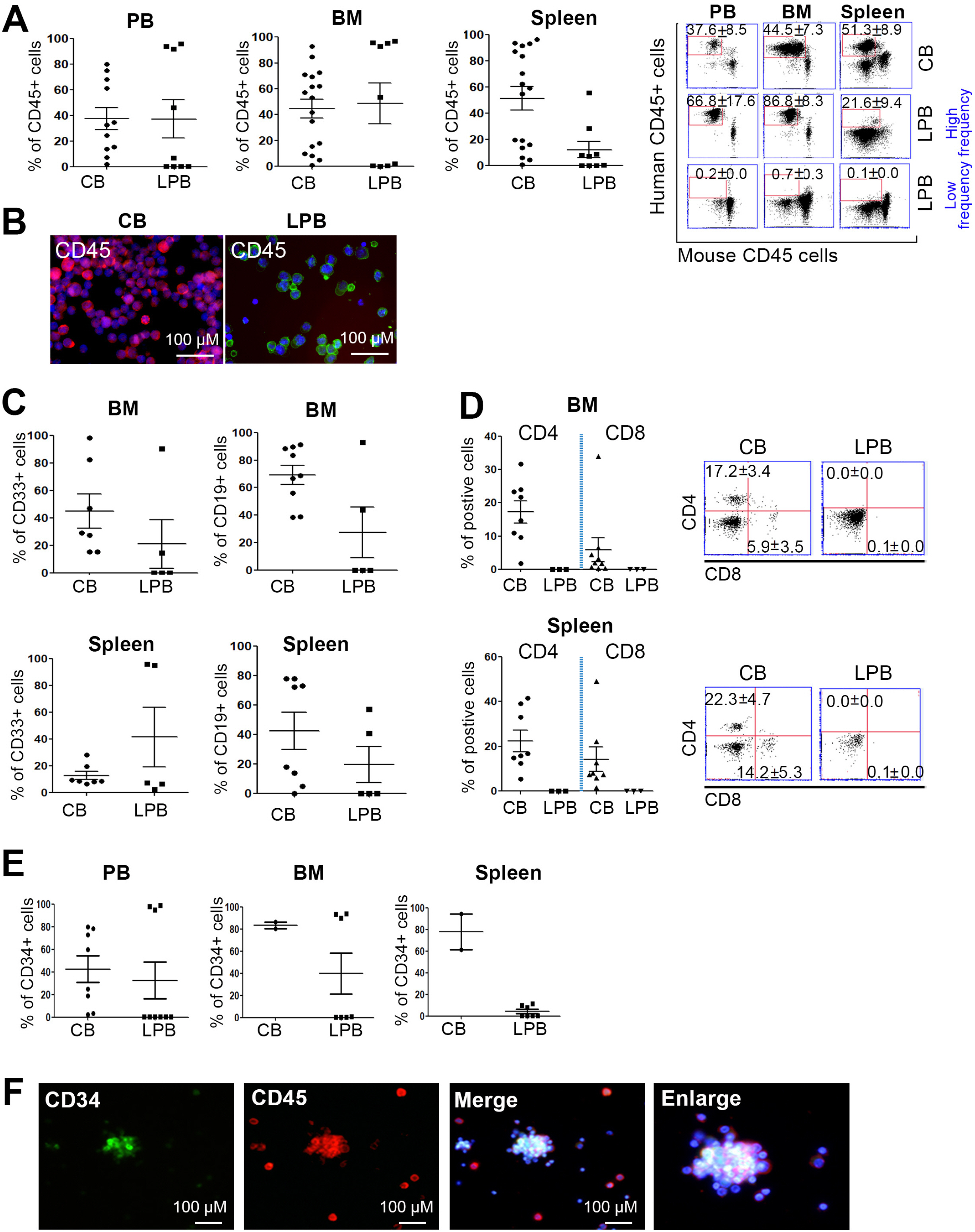
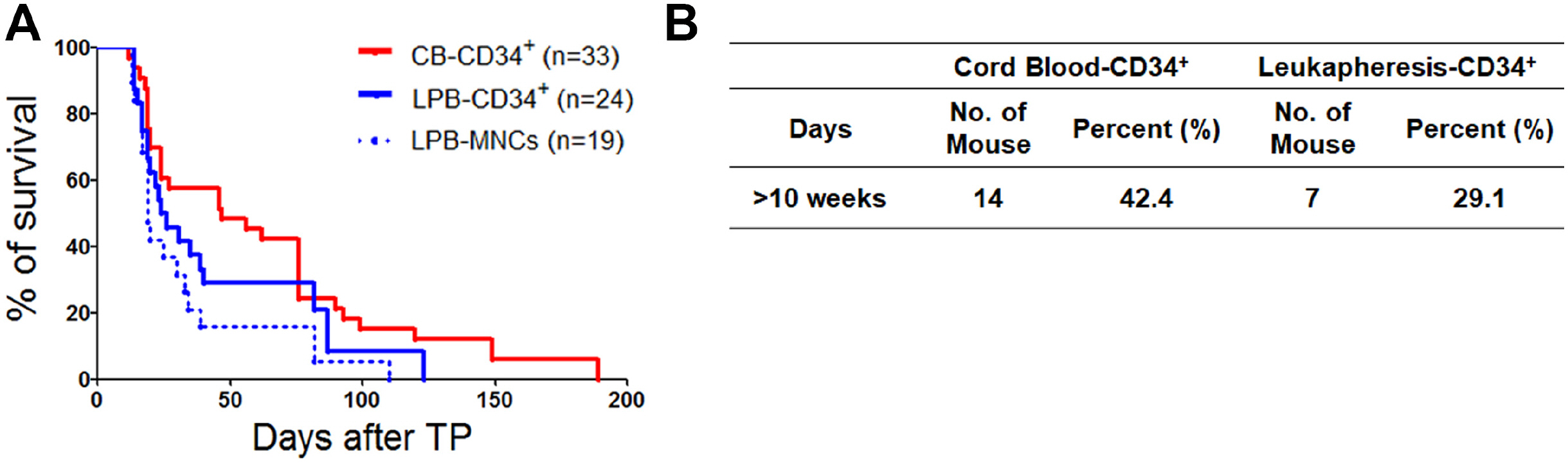
Human CD34+hematopoietic stem cells can reconstitute the human hematopoietic system when transplanted into immunocompromised mice after irradiation. Human leukapheresis peripheral blood (LPB)-and cord blood (CB)-derived CD34+ cells have a similar capacity to reconstitute myeloid lineage cells in a humanized mice (hu-mice) model. However, potent stem cells, such as CB-CD34+ cells, efficiently reconstitute the lymphoid system in vivo compared to LPB-CD34 + cells. Modeling the human hematolymphoid system is vital for studying immune cell crosstalk in human xenografted mice, with CB-CD34+ cells used as an optimized cell source because they are essential in reconstituting lymphoid lineage cells.
Methods and Results
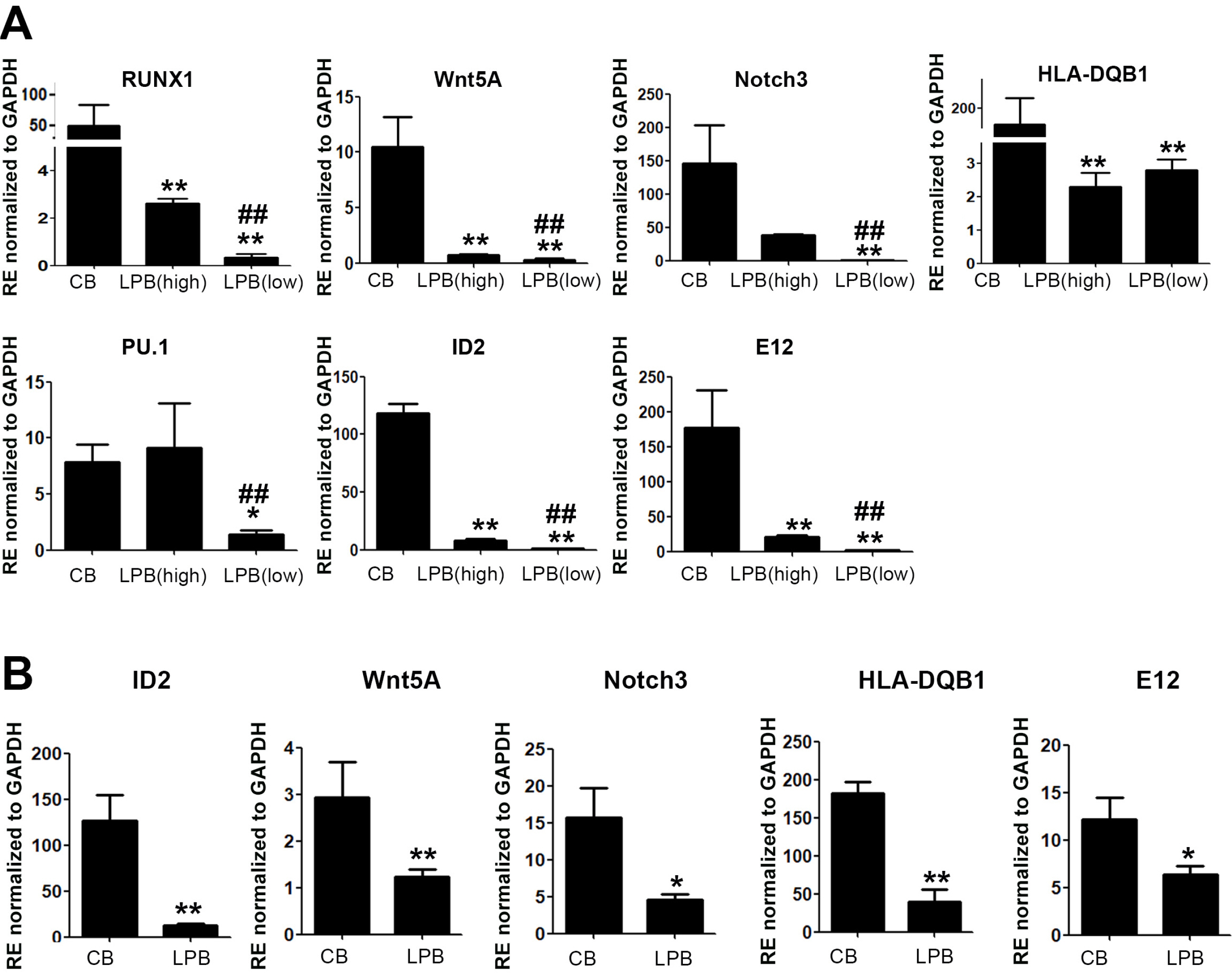
In this study, we established hu-mice that combined human characteristics with long-term survival and investigated the efficiency of the engraftment of lymphoid lineage cells derived from LPB- and CB-CD34+ cells in the bone marrow, spleen, and LPB. We found an overall increase in the transcriptional activity of lymphoid lineage genes in CB-CD34+ cells. Our results revealed that potent CB-CD34+ cells displaying a general upregulation of the expression of genes involved in lymphopoiesis could contribute to the hematolymphoid system in the humanized mice model with longevity.
Conclusions
Our data suggest that humanized mouse model by usage of CB-CD34 + cells displaying high expression of TFs for lymphoid lineage cells can contribute to study the immune response against lymphocytes.
Keyword
Figure
Reference
-
References
1. Allen TM, Brehm MA, Bridges S, Ferguson S, Kumar P, Mirochnitchenko O, Palucka K, Pelanda R, Sanders-Beer B, Shultz LD, Su L, PrabhuDas M. 2019; Humanized immune system mouse models: progress, challenges and opportunities. Nat Immunol. 20:770–774. DOI: 10.1038/s41590-019-0416-z. PMID: 31160798. PMCID: PMC7265413.
Article2. Shultz LD, Ishikawa F, Greiner DL. 2007; Humanized mice in translational biomedical research. Nat Rev Immunol. 7:118–130. DOI: 10.1038/nri2017. PMID: 17259968.
Article3. Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. 2002; NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 100:3175–3182. DOI: 10.1182/blood-2001-12-0207. PMID: 12384415.
Article4. Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. 2005; Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 174:6477–6489. DOI: 10.4049/jimmunol.174.10.6477. PMID: 15879151.
Article5. Volk V, Schneider A, Spineli LM, Grosshennig A, Stripecke R. 2016; The gender gap: discrepant human T-cell reconstitution after cord blood stem cell transplantation in humanized female and male mice. Bone Marrow Transplant. 51:596–597. DOI: 10.1038/bmt.2015.290. PMID: 26595075. PMCID: PMC4827003.
Article6. Volk V, Reppas AI, Robert PA, Spineli LM, Sundarasetty BS, Theobald SJ, Schneider A, Gerasch L, Deves Roth C, Klöss S, Koehl U, von Kaisenberg C, Figueiredo C, Hatzikirou H, Meyer-Hermann M, Stripecke R. 2017; Multidi-mensional analysis integrating human T-cell signatures in lymphatic tissues with sex of humanized mice for prediction of responses after dendritic cell immunization. Front Immunol. 8:1709. DOI: 10.3389/fimmu.2017.01709. PMID: 29276513. PMCID: PMC5727047.
Article7. Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. 2004; Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 304:104–107. DOI: 10.1126/science.1093933. PMID: 15064419.
Article8. Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. 2006; Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 108:487–492. DOI: 10.1182/blood-2005-11-4388. PMID: 16410443.
Article9. Jaiswal S, Pazoles P, Woda M, Shultz LD, Greiner DL, Brehm MA, Mathew A. 2012; Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology. 136:334–343. DOI: 10.1111/j.1365-2567.2012.03585.x. PMID: 22384859. PMCID: PMC3385033.
Article10. Schinnerling K, Rosas C, Soto L, Thomas R, Aguillón JC. 2019; Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front Immunol. 10:203. DOI: 10.3389/fimmu.2019.00203. PMID: 30837986. PMCID: PMC6389733.
Article11. Lubin I, Faktorowich Y, Lapidot T, Gan Y, Eshhar Z, Gazit E, Levite M, Reisner Y. 1991; Engraftment and development of human T and B cells in mice after bone marrow transplan-tation. Science. 252:427–431. DOI: 10.1126/science.1826797. PMID: 1826797.
Article12. Lee J, Li M, Milwid J, Dunham J, Vinegoni C, Gorbatov R, Iwamoto Y, Wang F, Shen K, Hatfield K, Enger M, Shafiee S, McCormack E, Ebert BL, Weissleder R, Yarmush ML, Parekkadan B. 2012; Implantable microenvironments to attract hematopoietic stem/cancer cells. Proc Natl Acad Sci U S A. 109:19638–19643. DOI: 10.1073/pnas.1208384109. PMID: 23150542. PMCID: PMC3511730.
Article13. Lee JY, Park S, Han AR, Lim J, Min WS, Kim HJ. 2014; High ALDHdim-expressing CD34+CD38-cells in leukapheresed peripheral blood is a reliable guide for a successful leukemic xenograft model of acute myeloid leukemia. Oncol Rep. 32:1638–1646. DOI: 10.3892/or.2014.3359. PMID: 25069538.
Article14. Lee JY, Han AR, Lee DR. 2019; T lymphocyte development and activation in humanized mouse model. Dev Reprod. 23:79–92. DOI: 10.12717/DR.2019.23.2.079. PMID: 31321348. PMCID: PMC6635618.
Article15. Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. 2011; Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood. 117:3076–3086. DOI: 10.1182/blood-2010-08-301507. PMID: 21252091. PMCID: PMC3062310.
Article16. Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA. 2014; Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 32:364–372. DOI: 10.1038/nbt.2858. PMID: 24633240. PMCID: PMC4017589.
Article17. Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. 2007; Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5:e201. DOI: 10.1371/journal.pbio.0050201. PMID: 17676974. PMCID: PMC1925137.
Article18. Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. 2005; Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 102:9194–9199. DOI: 10.1073/pnas.0503280102. PMID: 15967997. PMCID: PMC1153718.
Article19. Benita Y, Cao Z, Giallourakis C, Li C, Gardet A, Xavier RJ. 2010; Gene enrichment profiles reveal T-cell development, differentiation, and lineage-specific transcription factors including ZBTB25 as a novel NF-AT repressor. Blood. 115:5376–5384. DOI: 10.1182/blood-2010-01-263855. PMID: 20410506. PMCID: PMC2902135.
Article20. Lee JE, Lee JY, Park CH, Eum JH, Jung SK, Han AR, Seol DW, Lee JS, Shin HS, Im JH, Chun T, Ha K, Heo DR, Yoon TK, Lee DR. 2020; Cryopreserved human oocytes and cord blood cells can produce somatic cell nuclear transfer-de-rived pluripotent stem cells with a homozygous HLA type. Stem Cell Reports. 15:171–184. DOI: 10.1016/j.stemcr.2020.05.005. PMID: 32502464. PMCID: PMC7363744.
Article21. Shin SH, Lee JY, Lee TH, Park SH, Yahng SA, Yoon JH, Lee SE, Cho BS, Lee DG, Kim YJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS, Park CW, Kim HJ. 2015; SOCS1 and SOCS3 are expressed in mononuclear cells in human cytomegalovirus viremia after allogeneic hematopoietic stem cell transplantation. Blood Res. 50:40–45. DOI: 10.5045/br.2015.50.1.40. PMID: 25830129. PMCID: PMC4377337.
Article22. Booth CAG, Barkas N, Neo WH, Boukarabila H, Soilleux EJ, Giotopoulos G, Farnoud N, Giustacchini A, Ashley N, Carrelha J, Jamieson L, Atkinson D, Bouriez-Jones T, Prinjha RK, Milne TA, Teachey DT, Papaemmanuil E, Huntly BJP, Jacobsen SEW, Mead AJ. 2018; Ezh2 and Runx1 mutations collaborate to initiate lympho-myeloid leukemia in early thymic progenitors. Cancer Cell. 33:274–291.e8. DOI: 10.1016/j.ccell.2018.01.006. PMID: 29438697.
Article23. Lotem J, Levanon D, Negreanu V, Leshkowitz D, Friedlander G, Groner Y. 2013; Runx3-mediated transcriptional program in cytotoxic lymphocytes. PLoS One. 8:e80467. DOI: 10.1371/journal.pone.0080467. PMID: 24236182. PMCID: PMC3827420.
Article24. Valencia J, Martínez VG, Hidalgo L, Hernández-López C, Canseco NM, Vicente A, Varas A, Sacedón R. 2014; Wnt5a signaling increases IL-12 secretion by human dendritic cells and enhances IFN-γ production by CD4+ T cells. Immunol Lett. 162(1 Pt A):188–199. DOI: 10.1016/j.imlet.2014.08.015. PMID: 25196330.
Article25. Rozovski U, Harris DM, Li P, Liu Z, Jain P, Ferrajoli A, Burger JA, Bose P, Thompson PA, Jain N, Wierda WG, Uziel O, Keating MJ, Estrov Z. 2019; STAT3-induced Wnt5a provides chronic lymphocytic leukemia cells with survival advantage. J Immunol. 203:3078–3085. DOI: 10.4049/jimmunol.1900389. PMID: 31645416. PMCID: PMC6864283.
Article26. Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. 2003; Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 19:549–559. DOI: 10.1016/S1074-7613(03)00270-X.
Article27. Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. 2014; A committed precursor to innate lymphoid cells. Nature. 508:397–401. DOI: 10.1038/nature13047. PMID: 24509713. PMCID: PMC4003507.
Article28. Xu W, Cherrier DE, Chea S, Vosshenrich C, Serafini N, Petit M, Liu P, Golub R, Di Santo JP. 2019; An Id2RFP-reporter mouse redefines innate lymphoid cell precursor potentials. Immunity. 50:1054–1068.e3. DOI: 10.1016/j.immuni.2019.02.022. PMID: 30926235. PMCID: PMC6477155.
Article29. Boos MD, Yokota Y, Eberl G, Kee BL. 2007; Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 204:1119–1130. DOI: 10.1084/jem.20061959. PMID: 17452521. PMCID: PMC2118569.
Article30. van Galen P, Kreso A, Wienholds E, Laurenti E, Eppert K, Lechman ER, Mbong N, Hermans K, Dobson S, April C, Fan JB, Dick JE. 2014; Reduced lymphoid lineage priming promotes human hematopoietic stem cell expansion. Cell Stem Cell. 14:94–106. DOI: 10.1016/j.stem.2013.11.021. PMID: 24388174.
Article31. Cherrier M, Sawa S, Eberl G. 2012; Notch, Id2, and RORγt sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med. 209:729–740. DOI: 10.1084/jem.20111594. PMID: 22430492. PMCID: PMC3328368.
Article32. Bain G, Robanus Maandag EC, te Riele HP, Feeney AJ, Sheehy A, Schlissel M, Shinton SA, Hardy RR, Murre C. 1997; Both E12 and E47 allow commitment to the B cell lineage. Immunity. 6:145–154. DOI: 10.1016/S1074-7613(00)80421-5. PMID: 9047236.
Article33. Murre C. 2005; Helix-loop-helix proteins and lymphocyte develop-ment. Nat Immunol. 6:1079–1086. DOI: 10.1038/ni1260. PMID: 16239924.
Article34. Zhong C, Zhu J. 2017; Transcriptional regulators dictate innate lymphoid cell fates. Protein Cell. 8:242–254. DOI: 10.1007/s13238-017-0369-7. PMID: 28108952. PMCID: PMC5359184.
Article35. Laurenti E, Doulatov S, Zandi S, Plumb I, Chen J, April C, Fan JB, Dick JE. 2013; The transcriptional architecture of early human hematopoiesis identifies multilevel control of lymphoid commitment. Nat Immunol. 14:756–763. DOI: 10.1038/ni.2615. PMID: 23708252. PMCID: PMC4961471.
Article36. Pouzolles M, Oburoglu L, Taylor N, Zimmermann VS. 2016; Hematopoietic stem cell lineage specification. Curr Opin Hematol. 23:311–317. DOI: 10.1097/MOH.0000000000000260. PMID: 27135980.
Article37. Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, Watson CJ, Delriviere L, Alexander GJ, Jamieson NV, Bradley JA, Taylor CJ. 2004; Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Trans-plantation. 77:441–446. DOI: 10.1097/01.TP.0000103721.29729.FE. PMID: 14966423.
Article38. Jamil MO, Mineishi S. 2015; State-of-the-art acute and chronic GVHD treatment. Int J Hematol. 101:452–466. DOI: 10.1007/s12185-015-1785-1. PMID: 25864189.
Article39. Minoda Y, Virshup I, Leal Rojas I, Haigh O, Wong Y, Miles JJ, Wells CA, Radford KJ. 2017; Human CD141+ dendritic cell and CD1c+ dendritic cell undergo concordant early genetic programming after activation in humanized mice in vivo. Front Immunol. 8:1419. DOI: 10.3389/fimmu.2017.01419. PMID: 29163495. PMCID: PMC5670352.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Etv5, a transcription factor with versatile functions in male reproduction
- SoxD Transcription Factors: Multifaceted Players of Neural Development
- Early Growth Response-1 Plays a Non-redundant Role in the Differentiation of B Cells into Plasma Cells
- Lineage Differentiation Program of Invariant Natural Killer T Cells
- Anti-proliferative Activity of T-bet