Ann Pediatr Endocrinol Metab.
2021 Mar;26(1):46-52. 10.6065/apem.2040110.055.
Novel function of adrenocorticotropic hormone in the stimulation of vascular endothelial growth factor release in healthy children and adolescents: a proof-of-concept study
- Affiliations
-
- 1Nationwide Children's Hospital, Columbus, OH, USA
- 2Akron Children's Hospital, Akron, OH, USA
- KMID: 2514152
- DOI: http://doi.org/10.6065/apem.2040110.055
Abstract
- Purpose
To assess the effect of adrenocorticotropic hormone (ACTH) on plasma vascular endothelial growth factor (VEGF) levels in healthy children and adolescents and to inform future work on the effects of ACTH on VEGF in bone.
Methods
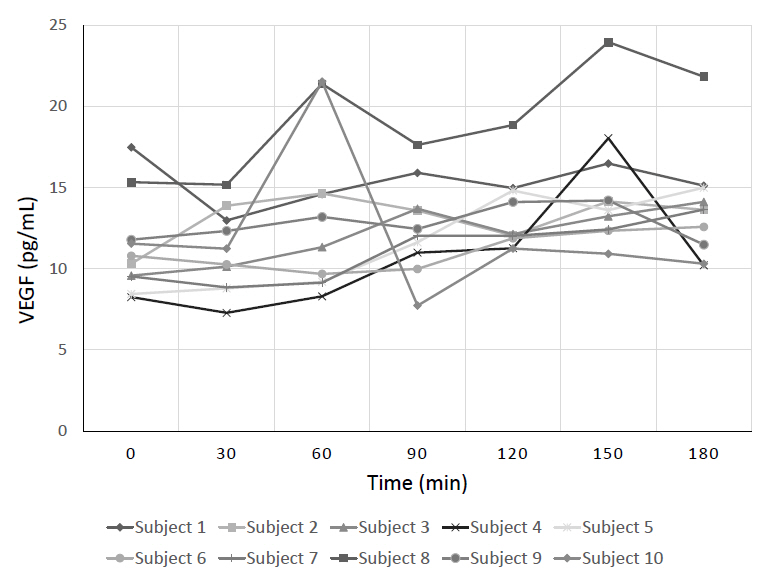
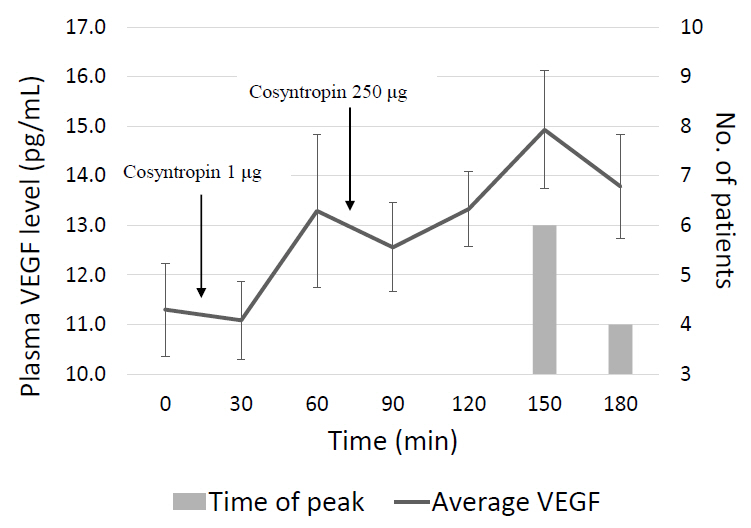
An Institutional Review Board-approved prospective study of 10 healthy subjects, ages 9–17, was conducted to assess the effect of ACTH on plasma VEGF levels. VEGF levels were collected at baseline and every 30 minutes for 3 hours. Cosyntropin (a synthetic ACTH analogue) was administered at a low-dose (1 μg) given at t=0 minutes and a high-dose (250 μg) given at t=60 minutes. A Friedman test was performed comparing baseline to peak VEGF levels after stimulation with low-dose and high-dose cosyntropin.
Results
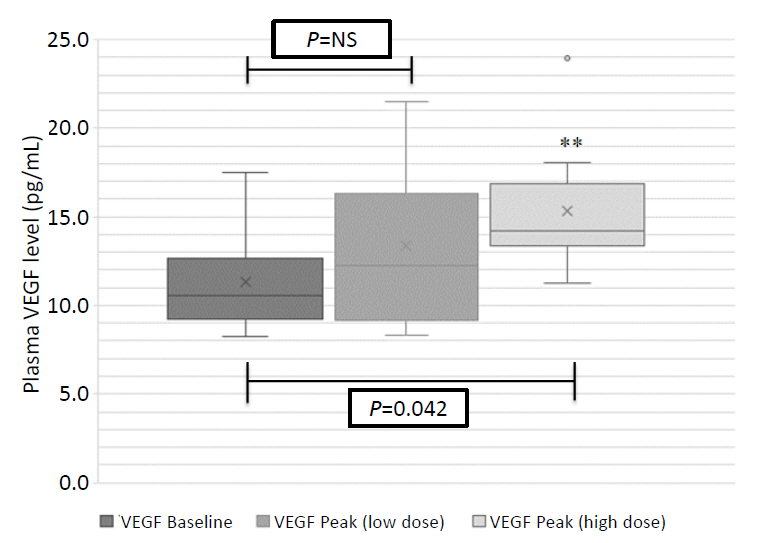
Peak plasma VEGF levels significantly increased after high-dose cosyntropin compared with baseline (P=0.042). Peak plasma VEGF levels did not significantly increase after low-dose cosyntropin compared to baseline.
Conclusion
To our knowledge, this is the first study to demonstrate that ACTH administration causes a significant increase in plasma VEGF levels in humans. This finding may have important implications in the protective effects of ACTH on bone. Decreased bone mineral density and adrenal suppression are common side effects of glucocorticoid use in pediatrics. VEGF increases vascularity and may play a role in reducing glucocorticoid-induced bone disease. Animal studies have shown that ACTH stimulates release of VEGF in osteoblasts, though this effect has yet to be evaluated in humans.
Keyword
Figure
Reference
-
References
1. Leonard MB. Glucocorticoid-induced osteoporosis in children: impact of the underlying disease. Pediatrics. 2007; 119 Suppl 2:S166–74.
Article2. Aulakh R, Singh S. Strategies for minimizing corticosteroid toxicity: a review. Indian J Pediatr. 2008; 75:1067–73.
Article3. Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006; 17:144–9.
Article4. Xie XH, Wang XL, Yang HL, Zhao DW, Qin L. Steroid-associated osteonecrosis: Epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview). J Orthop Translat. 2015; 3:58–70.
Article5. Yang YQ, Tan YY, Wong R, Wenden A, Zhang LK, Rabie AB. The role of vascular endothelial growth factor in ossification. Int J Oral Sci. 2012; 4:64–8.
Article6. Zaidi M, Sun L, Liu P, Davies TF, New M, Zallone A, et al. Pituitary-bone connection in skeletal regulation. Horm Mol Biol Clin Investig. 2016; 28:85–94.
Article7. Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann N Y Acad Sci. 2010; 1192:110–6.
Article8. Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad S ci U S A. 2010; 107:8782–7.
Article9. Wang G, Zhang CQ, Sun Y, Feng Y, Chen SB, Cheng XG, et al. Changes in femoral head blood supply and vascular endothelial growth factor in rabbits with steroid-induced osteonecrosis. J Int Med Res. 2010; 38:1060–9.
Article10. Tourkova IL, Liu L, Sutjarit N, Larrouture QC, Luo J, Robinson LJ, et al. Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D3 enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab Invest. 2017; 97:1072–83.
Article11. Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015; 99:137–48.
Article12. Thaler LM, Blevins LS. The low dose (1-microg) adrenocorticotropin stimulation test in the evaluation of patients with suspected central adrenal insufficiency. J Clin Endocrinol Metab. 1998; 83:2726–9.13. Hamilton DD, Cotton BA. Cosyntropin as a diagnostic agent in the screening of patients for adrenocortical insufficiency. Clin Pharmacol. 2010; 2:77–82.14. El-Farhan N, Pickett A, Ducroq D, Bailey C, Mitchem K, Morgan N, et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol (Oxf). 2013; 78:673–80.
Article15. Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2016; 101:364–89.
Article16. Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res. 2006; 23:312–28.
Article17. Dassan P, Keir G, Jager HR, Brown MM. Value of measuring serum vascular endothelial growth factor levels in diagnosing acute ischemic stroke. Int J Stroke. 2012; 7:454–9.
Article18. Meo S, Dittadi R, Gion M. Biological variation of vascular endothelial growth factor. Clin Chem Lab Med. 2005; 43:342–3.
Article19. Mallet C, Feraud O, Ouengue-Mbele G, Gaillard I, Sappay N, Vittet D, et al. Differential expression of VEGF receptors in adrenal atrophy induced by dexamethasone: a protective role of ACTH. Am J Physiol Endocrinol Metab. 2003; 284:E156–67.
Article20. Lefebvre H, Thomas M, Duparc C, Bertherat J, Louiset E. Role of ACTH in the Interactive/paracrine regulation of adrenal steroid secretion in physiological and pathophysiological conditions. Front Endocrinol (Lausanne). 2016; 7:98.
Article21. Minetto M, Reimondo G, Osella G, Ventura M, Angeli A, Terzolo M. Bone loss is more severe in primary adrenal than in pituitar y-dependent Cushing's syndrome. Osteoporos Int. 2004; 15:855–61.
Article22. Guo W, Li F, Zhu C, Wang B, Wang K, Dai C, et al. Effect of hypercortisolism on bone mineral density and bone metabolism: A potential protective effect of adrenocorticotropic hormone in patients with Cushing's disease. J Int Med Res. 2018; 46:492–503.
Article23. Elias LL, Huebner A, Metherell LA, Canas A, Warne GL, Bitti ML, et al. Tall stature in familial glucocorticoid deficiency. Clin Endocrinol (Oxf). 2000; 53:423–30.
Article24. Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2011; 8:122–8.
Article25. Montero-Melendez T. ACTH: The forgotten therapy. Semin Immunol. 2015; 27:216–26.
Article26. Lieberman KV, Pavlova-Wolf A. Adrenocorticotropic hormone therapy for the treatment of idiopathic nephrotic syndrome in children and young adults: a systematic review of early clinical studies with contemporary relevance. J Nephrol. 2017; 30:35–44.
Article27. Tumlin JA, Galphin CM, Rovin BH. Advanced diabetic nephropathy with nephrotic range proteinuria: a pilot study of the long-term efficacy of subcutaneous ACTH gel on proteinuria, progression of CKD, and urinary levels of VEGF and MCP-1. J Diabetes Res. 2013; 2013:489869.
Article28. Lankhorst S, Baelde HJ, Verstijnen J, Ten Tije AJ, Thelen MHM, Danser AHJ, et al. Cumulative dose of bevacizumab associates with albuminuria rather than podocyturia in cancer patients. J Am Soc Hypertens. 2018; 12:e1–7.
Article29. Tordjman K, Jaffe A, Trostanetsky Y, Greenman Y, Limor R, Stern N. Low-dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo-pituitary-adrenal axis function: sensitivity, specificity and accuracy in comparison with the high-dose (250 microgram) test. Clin Endocrinol (Oxf). 2000; 52:633–40.30. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003; 139:194–204.
Article31. Ospina NS, Al Nofal A, Bancos I, Javed A, Benkhadra K, Kapoor E, et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016; 101:427–34.
Article32. Wade M, Baid S, Calis K, Raff H, Sinaii N, Nieman L. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur J Endocrinol. 2010; 162:109–13.33. Gettig J, Cummings JP, Matuszewski K. H.p. Acthar gel and cosyntropin review: clinical and financial implications. P T. 2009; 34:250–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The study of serum adrenocorticotropic hormone and cortisol level, applying transcutaneous electrical nerve stimulation to the meridian and non-meridian points
- Diagnosis and Treatment of Hypopituitarism
- Expression of Vascular Endothelial Growth Factor : Clinical Implications in Cervical Neoplasia
- Dexamethasone Attenuates PDGF- and TGF-beta-enhanced Vascular Endothelial Growth Factor Production in Cultured Human Bronchial Smooth Muscle Cells
- Transforming growth factor-beta promoted vascular endothelial growth factor release by human lung fibroblasts