Endocrinol Metab.
2021 Feb;36(1):12-21. 10.3803/EnM.2021.101.
The Genotype-Based Morphology of Aldosterone-Producing Adrenocortical Disorders and Their Association with Aging
- Affiliations
-
- 1Department of Pathology, Tohoku University Graduate School of Medicine, Sendai, Japan
- 2Division of Clinical Hypertension, Endocrinology and Metabolism, Tohoku University Graduate School of Medicine, Sendai, Japan
- 3Division of Nephrology, Endocrinology, and Vascular Medicine, Tohoku University Hospital, Sendai, Japan
- 4Division of Pathology, Faculty of Medicine, Tohoku Medical and Pharmaceutical University, Sendai, Japan
- KMID: 2513283
- DOI: http://doi.org/10.3803/EnM.2021.101
Abstract
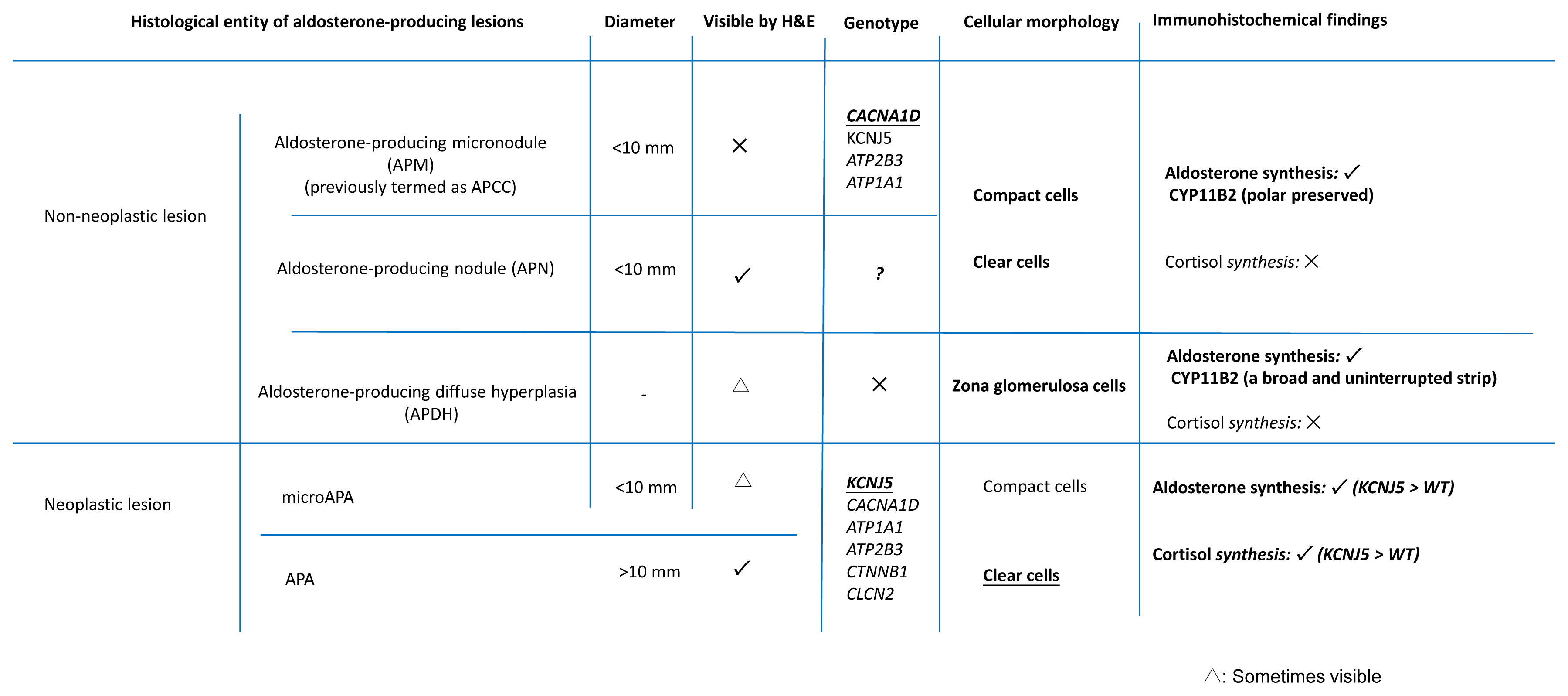
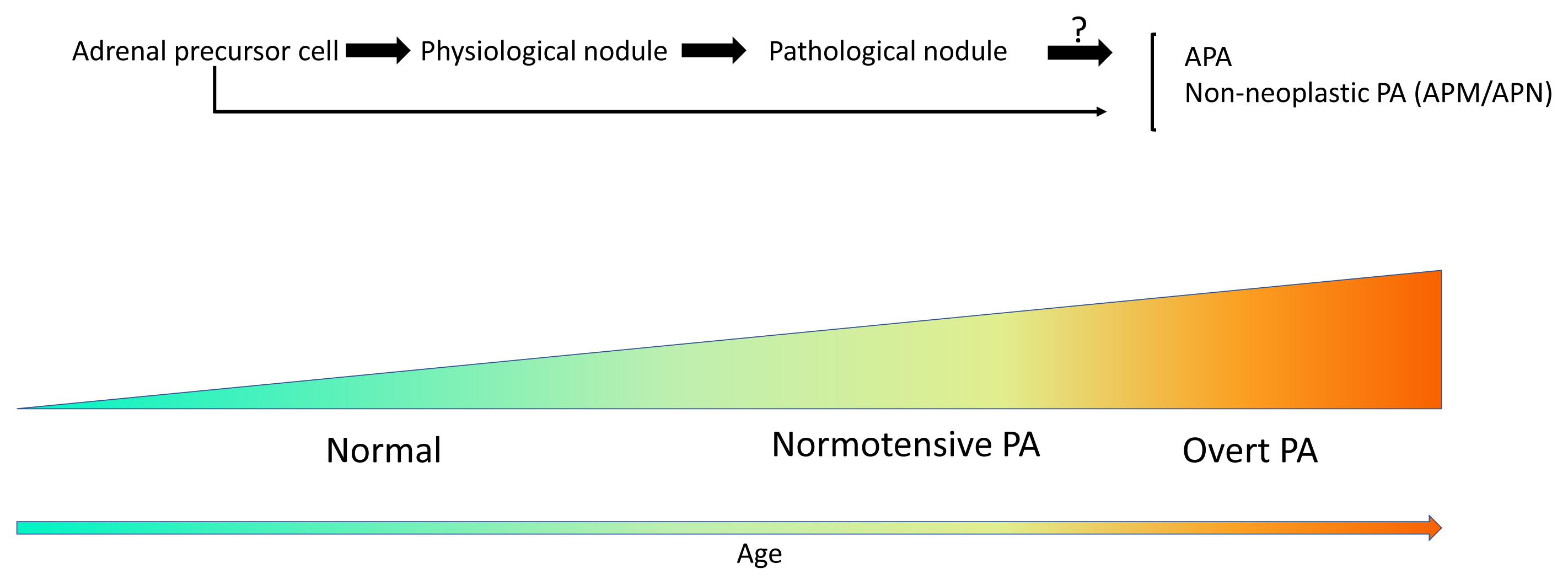
- Primary aldosteronism (PA) is the most common cause of secondary hypertension, and is associated with an increased incidence of cardiovascular events. PA itself is clinically classified into the following two types: unilateral PA, mostly composed of aldosteroneproducing adenoma (APA); and bilateral hyperaldosteronism, consisting of multiple aldosterone-producing micronodules (APMs) and aldosterone-producing diffuse hyperplasia. Histopathologically, those disorders above are all composed of compact and clear cells. The cellular morphology in the above-mentioned aldosterone-producing disorders has been recently reported to be closely correlated with patterns of somatic mutations of ion channels including KCNJ5, CACNA1D, ATP1A1, ATP2B3, and others. In addition, in non-pathological adrenal glands, APMs are frequently detected regardless of the status of the renin-angiotensin-aldosterone system (RAAS). Aldosterone-producing nodules have been also proposed as non-neoplastic nodules that can be identified by hematoxylin and eosin staining. These non-neoplastic CYP11B2-positive nodules could represent possible precursors of APAs possibly due to the presence of somatic mutations. On the other hand, aging itself also plays a pivotal role in the development of aldosterone-producing lesions. For instance, the number of APMs was also reported to increase with aging. Therefore, recent studies indicated the novel classification of PA into normotensive PA (RAAS-independent APM) and clinically overt PA.
Keyword
Figure
Reference
-
1. Funder JW. Medicine: the genetics of primary aldosteronism. Science. 2011; 331:685–6.2. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006; 48:2293–300.
Article3. Williams JS, Williams GH, Raji A, Jeunemaitre X, Brown NJ, Hopkins PN, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006; 20:129–36.
Article4. Byrd JB, Turcu AF, Auchus RJ. Primary aldosteronism: practical approach to diagnosis and management. Circulation. 2018; 138:823–35.5. Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010; 95:2296–305.
Article6. Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021; 106:42–54.
Article7. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004; 136:1227–35.
Article8. Toniato A, Bernante P, Rossi GP, Pelizzo MR. The role of adrenal venous sampling in the surgical management of primary aldosteronism. World J Surg. 2006; 30:624–7.
Article9. Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018; 72:874–80.
Article10. Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017; 102:1182–92.11. Omata K, Anand SK, Hovelson DH, Liu CJ, Yamazaki Y, Nakamura Y, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017; 1:787–99.
Article12. Nishimoto K, Seki T, Kurihara I, Yokota K, Omura M, Nishikawa T, et al. Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J Clin Endocrinol Metab. 2016; 101:6–9.
Article13. Hegstad R, Brown RD, Jiang NS, Kao P, Weinshilboum RM, Strong C, et al. Aging and aldosterone. Am J Med. 1983; 74:442–8.
Article14. Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011; 331:768–72.
Article15. Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013; 45:1055–60.
Article16. Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013; 45:1050–4.
Article17. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013; 45:440–4.
Article18. Akerstrom T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016; 6:19546.
Article19. Dutta RK, Arnesen T, Heie A, Walz M, Alesina P, Soderkvist P, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019; 181:K37–41.
Article20. Cheng CJ, Sung CC, Wu ST, Lin YC, Sytwu HK, Huang CL, et al. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J Clin Endocrinol Metab. 2015; 100:E155–63.21. Felizola SJ, Maekawa T, Nakamura Y, Satoh F, Ono Y, Kikuchi K, et al. Voltage-gated calcium channels in the human adrenal and primary aldosteronism. J Steroid Biochem Mol Biol. 2014; 144(Pt B):410–6.
Article22. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014; 64:354–61.
Article23. Di Leva F, Domi T, Fedrizzi L, Lim D, Carafoli E. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys. 2008; 476:65–74.
Article24. Bonnet-Serrano F, Bertherat J. Genetics of tumors of the adrenal cortex. Endocr Relat Cancer. 2018; 25:R131–52.
Article25. Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015; 4:e06315.
Article26. Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, et al. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine. 2016; 13:225–36.
Article27. Murthy M, Xu S, Massimo G, Wolley M, Gordon RD, Stowasser M, et al. Role for germline mutations and a rare coding single nucleotide polymorphism within the KCNJ5 potassium channel in a large cohort of sporadic cases of primary aldosteronism. Hypertension. 2014; 63:783–9.
Article28. Korah HE, Scholl UI. An update on familial hyperaldosteronism. Horm Metab Res. 2015; 47:941–6.
Article29. Stowasser M, Bachmann AW, Huggard PR, Rossetti TR, Gordon RD. Severity of hypertension in familial hyperaldosteronism type I: relationship to gender and degree of biochemical disturbance. J Clin Endocrinol Metab. 2000; 85:2160–6.
Article30. Lifton RP, Dluhy RG, Powers M, Ulick S, Lalouel JM. The molecular basis of glucocorticoid-remediable aldosteronism, a Mendelian cause of human hypertension. Trans Assoc Am Physicians. 1992; 105:64–71.31. Stowasser M, Wolley M, Wu A, Gordon RD, Schewe J, Stolting G, et al. Pathogenesis of familial hyperaldosteronism type II: new concepts involving anion channels. Curr Hypertens Rep. 2019; 21:31.
Article32. Fernandes-Rosa FL, Daniil G, Orozco IJ, Goppner C, El Zein R, Jain V, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018; 50:355–61.
Article33. Nanba K, Omata K, Else T, Beck PC, Nanba AT, Turcu AF, et al. Targeted molecular characterization of aldosterone-producing adenomas in White Americans. J Clin Endocrinol Metab. 2018; 103:3869–76.
Article34. De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020; 75:1034–44.
Article35. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015; 22:191–200.
Article36. Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, et al. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab. 2013; 98:1567–74.
Article37. Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015; 112:E4591–9.
Article38. Yamazaki Y, Omata K, Tezuka Y, Ono Y, Morimoto R, Adachi Y, et al. Tumor cell subtypes based on the intracellular hormonal activity in KCNJ5-mutated aldosterone-producing adenoma. Hypertension. 2018; 72:632–40.
Article39. Gao X, Yamazaki Y, Tezuka Y, Pieroni J, Ishii K, Atsumi N, et al. Intratumoral heterogeneity of the tumor cells based on in situ cortisol excess in cortisol-producing adenomas: an association among morphometry, genotype and cellular senescence. J Steroid Biochem Mol Biol. 2020; 204:105764.
Article40. Neville AM, O’Hare MJ. The human adrenal cortex. Pathology and biology: an integrated approach. Berlin: Springer;1982.
Article41. Tsuchiyama H, Kawai K, Harada T, Shigematsu K, Sugihara H. Functional pathology of aldosterone-producing adenoma. Acta Pathol Jpn. 1980; 30:967–76.
Article42. Ono Y, Yamazaki Y, Omata K, Else T, Tomlins SA, Rhayem Y, et al. Histological characterization of aldosterone-producing adrenocortical adenomas with different somatic mutations. J Clin Endocrinol Metab. 2020; 105:e282–9.
Article43. Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012; 97:E819–29.44. Gioco F, Seccia TM, Gomez-Sanchez EP, Rossi GP, Gomez-Sanchez CE. Adrenal histopathology in primary aldosteronism: is it time for a change? Hypertension. 2015; 66:724–30.45. Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010; 56:885–92.
Article46. Boulkroun S, Samson-Couterie B, Golib-Dzib JF, Amar L, Plouin PF, Sibony M, et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011; 152:4753–63.
Article47. Hammer GD, Basham KJ. Stem cell function and plasticity in the normal physiology of the adrenal cortex. Mol Cell Endocrinol. 2021; 519:111043.
Article48. Freedman BD, Kempna PB, Carlone DL, Shah M, Guagliardo NA, Barrett PQ, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013; 26:666–73.
Article49. Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, et al. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017; 167:630–41.
Article50. Weidmann P, De Myttenaere-Bursztein S, Maxwell MH, de Lima J. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int. 1975; 8:325–33.51. Noth RH, Lassman MN, Tan SY, Fernandez-Cruz A Jr, Mulrow PJ. Age and the renin-aldosterone system. Arch Intern Med. 1977; 137:1414–7.
Article52. Tsunoda K, Abe K, Goto T, Yasujima M, Sato M, Omata K, et al. Effect of age on the renin-angiotensin-aldosterone system in normal subjects: simultaneous measurement of active and inactive renin, renin substrate, and aldosterone in plasma. J Clin Endocrinol Metab. 1986; 62:384–9.
Article53. Laragh JH, Sealey JE. The plasma renin test reveals the contribution of body sodium-volume content (V) and renin-angiotensin (R) vasoconstriction to long-term blood pressure. Am J Hypertens. 2011; 24:1164–80.
Article54. Rakotondrazafy J, Brudieux R. Age-related change in plasma aldosterone response to exogenous angiotensin II in the rat. Horm Res. 1993; 39:156–60.
Article55. Giacche M, Vuagnat A, Hunt SC, Hopkins PN, Fisher ND, Azizi M, et al. Aldosterone stimulation by angiotensin II: influence of gender, plasma renin, and familial resemblance. Hypertension. 2000; 35:710–6.56. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017; 136:347–55.
Article57. Hornsby PJ. Aging of the human adrenal cortex. Ageing Res Rev. 2002; 1:229–42.
Article58. Aiba M, Fujibayashi M. Alteration of subcapsular adrenocortical zonation in humans with aging: the progenitor zone predominates over the previously well-developed zona glomerulosa after 40 years of age. J Histochem Cytochem. 2011; 59:557–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aldosterone-Producing Adrenocortical Carcinoma without Hypertension
- A Case Report of an Aldosterone-producing Adrenocortical Carcinoma
- Two Case of Primary Aldosteronism Induced by Aldosterone Producing Adrenal Adenoma in a Family
- A Case of a Coexisting Aldosterone-Producing Adrenal Adenoma and Ipsilateral Renal Artery Stenosis
- A case of adrenocortical adenoma with primary hyperaldosteronism and subclinical Cushing's syndrome