Biomarker-Guided Risk Assessment for Acute Kidney Injury: Time for Clinical Implementation?
- Affiliations
-
- 1Medical Faculty, University Clinic for Cardiology and Angiology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany
- 2Diaverum Renal Services, MVZ Potsdam, Potsdam, Germany
- 3Medical Faculty, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
- 4Department of Nephrology and Endocrinology, Klinikum Ernst von Bergmann, Potsdam, Germany
- 5Department of Medical Biometry and Epidemiology, University Medical Center HamburgEppendorf, Germany
- 6Department of Cardiology, Immanuel Diakonie Bernau, Heart Center Brandenburg, Brandenburg Medical School Theodor Fontane (MHB), Germany
- 7Institute of Social Medicine and Health Systems Research, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
- 8Faculty of Health Sciences Brandenburg, Potsdam, Germany
- KMID: 2512729
- DOI: http://doi.org/10.3343/alm.2021.41.1.1
Abstract
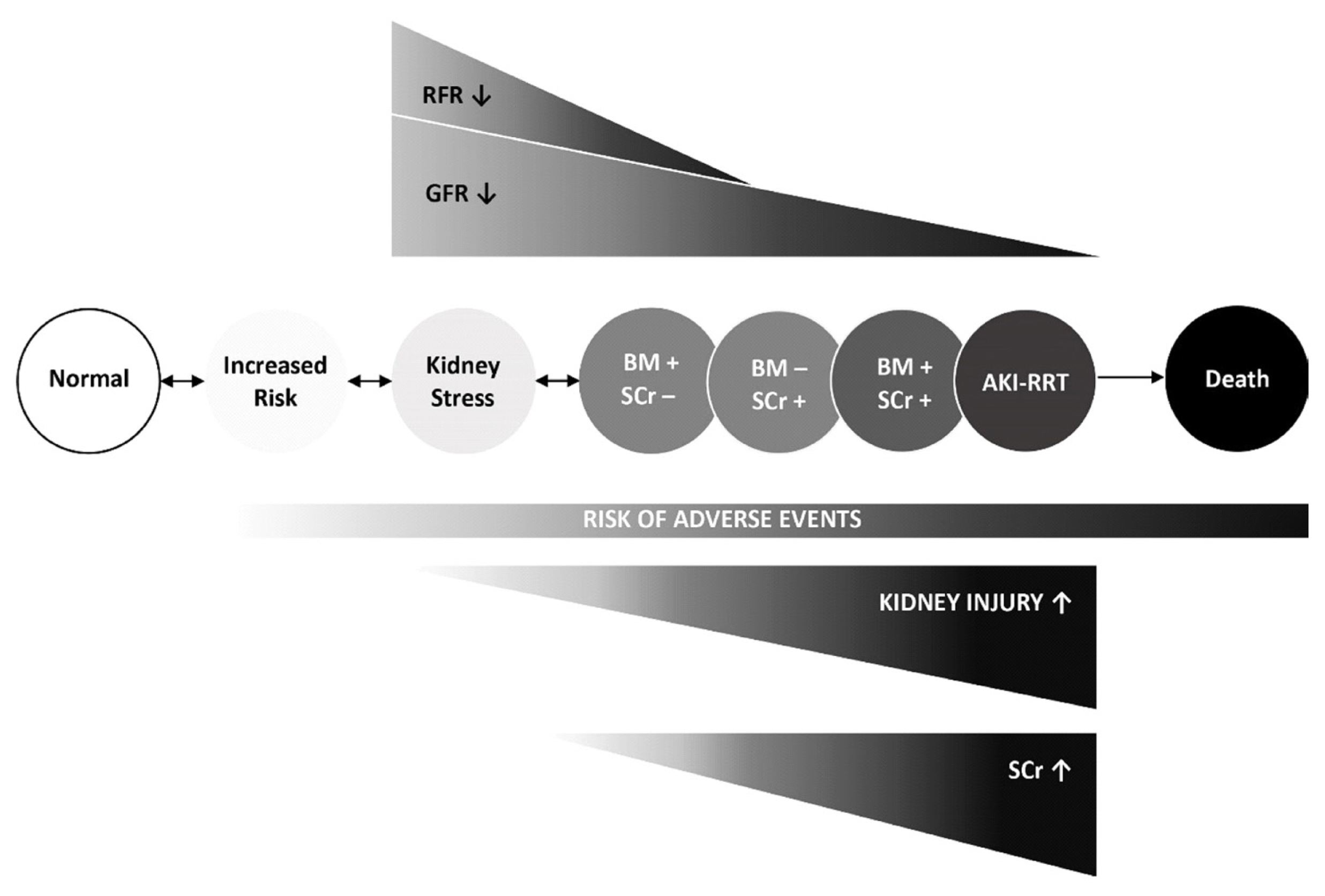
- Acute kidney injury (AKI) is a common and serious complication in hospitalized patients, which continues to pose a clinical challenge for treating physicians. The most recent Kidney Disease Improving Global Outcomes practice guidelines for AKI have restated the importance of earliest possible detection of AKI and adjusting treatment accordingly. Since the emergence of initial studies examining the use of neutrophil gelatinase-associated lipocalin (NGAL) and cycle arrest biomarkers, tissue inhibitor metalloproteinase-2 (TIMP-2) and insulin-like growth factor-binding protein (IGFBP7), for early diagnosis of AKI, a vast number of studies have investigated the accuracy and additional clinical benefits of these biomarkers. As proposed by the Acute Dialysis Quality Initiative, new AKI diagnostic criteria should equally utilize glomerular function and tubular injury markers for AKI diagnosis. In addition to refining our capabilities in kidney risk prediction with kidney injury biomarkers, structural disorder phenotypes referred to as “preclinical-” and “subclinical AKI” have been described and are increasingly recognized. Additionally, positive biomarker test findings were found to provide prognostic information regardless of an acute decline in renal function (positive serum creatinine criteria). We summarize and discuss the recent findings focusing on two of the most promising and clinically available kidney injury biomarkers, NGAL and cell cycle arrest markers, in the context of AKI phenotypes. Finally, we draw conclusions regarding the clinical implications for kidney risk prediction.
Keyword
Figure
Cited by 4 articles
-
Predictive Value of Plasma NGAL:Hepcidin-25 for Major Adverse Kidney Events After Cardiac Surgery with Cardiopulmonary Bypass: A Pilot Study
Christian Albert, Michael Haase, Annemarie Albert, Martin Ernst, Siegfried Kropf, Rinaldo Bellomo, Sabine Westphal, Rüdiger C. Braun-Dullaeus, Anja Haase-Fielitz, Saban Elitok
Ann Lab Med. 2021;41(4):357-365. doi: 10.3343/alm.2021.41.4.357.Biomarker Rule-in or Rule-out in Patients With Acute Diseases for Validation of Acute Kidney Injury in the Emergency Department (BRAVA): A Multicenter Study Evaluating Urinary TIMP-2/IGFBP7
Hyun Suk Yang, Mina Hur, Kyeong Ryong Lee, Hanah Kim, Hahn Young Kim, Jong Won Kim, Mui Teng Chua, Win Sen Kuan, Horng Ruey Chua, Chagriya Kitiyakara, Phatthranit Phattharapornjaroen, Anchalee Chittamma, Thiyapha Werayachankul, Urmila Anandh, Sanjeeva Herath, Zoltan Endre, Andrea Rita Horvath, Paola Antonini, Salvatore Di Somma
Ann Lab Med. 2022;42(2):178-187. doi: 10.3343/alm.2022.42.2.178.New Issues With Neutrophil Gelatinase-associated Lipocalin in Acute Kidney Injury
Sun Young Cho, Mina Hur
Ann Lab Med. 2023;43(6):529-530. doi: 10.3343/alm.2023.43.6.529.Neutrophil Gelatinase-Associated Lipocalin Cutoff Value Selection and Acute Kidney Injury Classification System Determine Phenotype Allocation and Associated Outcomes
Annemarie Albert, Sebastian Radtke, Louisa Blume, Rinaldo Bellomo, Michael Haase, Philipp Stieger, Ulrich Paul Hinkel, Rüdiger C. Braun-Dullaeus, Christian Albert
Ann Lab Med. 2023;43(6):539-553. doi: 10.3343/alm.2023.43.6.539.
Reference
-
1. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005; 16:3365–70.
Article2. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019; 394:1949–64.
Article3. Haase-Fielitz A, Ernst M, Lehmanski F, Gleumes J, Blödorn G, Spura A, et al. Treatment, clinical course, and cross-sectoral information transmission in patients with acute-on-chronic kidney injury. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. 2019; 62:773–81.4. Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: improving global outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012; 2:1–138.5. Kashani K, Rosner MH, Haase M, Lewington AJP, O’Donoghue DJ, Wilson FP, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019; 14:941–53.
Article6. Haase M, Kribben A, Zidek W, Floege J, Albert C, Isermann B, et al. Electronic alerts for acute kidney injury. Dtsch Arztebl Int. 2017; 114:1–8.
Article7. Haase-Fielitz A, Elitok S, Schostak M, Ernst M, Isermann B, Albert C, et al. The effects of intensive versus routine treatment in patients with acute kidney injury. Dtsch Arztebl Int. 2020; 117:289–96.
Article8. McCullough PA, Shaw AD, Haase M, Bouchard J, Waikar SS, Siew ED, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013; 182:13–29.
Article9. Huen SC, Parikh CR. Molecular phenotyping of clinical AKI with novel urinary biomarkers. Am J Physiol Renal Physiol. 2015; 309:F406–13.
Article10. Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011; 57:1752–61.11. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009; 20:672–9.
Article12. Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016; 8:E305–11.
Article13. Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012; 23:13–21.
Article14. Kim H, Hur M, Lee S, Marino R, Magrini L, Cardelli P, et al. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Ann Lab Med. 2017; 37:388–97.
Article15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014; 9:12–20.
Article16. Ji M, Lee YH, Hur M, Kim H, Cho HI, Yang HS, et al. Comparing results of five glomerular filtration rate-estimating equations in the Korean general population: MDRD study, revised Lund-Malmö, and three CKD-EPI equations. Ann Lab Med. 2016; 36:521–8.
Article17. Wang X, Lin X, Xie B, Huang R, Yan Y, Liu S, et al. Early serum cystatin C-enhanced risk prediction for acute kidney injury post cardiac surgery: a prospective, observational, cohort study. Biomarkers. 2020; 25:20–6.
Article18. Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014; 64:2753–62.
Article19. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008; 51:395–406.
Article20. Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, Möckel M, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg. 2009; 88:124–30.
Article21. Correa S, Morrow DA, Braunwald E, Davies RY, Goodrich EL, Murphy SA, et al. Cystatin C for risk stratification in patients after an acute coronary syndrome. J Am Heart Assoc. 2018; 7:e009077.
Article22. Flores-Blanco PJ, Manzano-Fernández S, Pérez-Calvo JI, Pastor-Pérez FJ, Ruiz-Ruiz FJ, Carrasco-Sánchez FJ, et al. Cystatin C-based CKD-EPI equations and N-terminal pro-B-type natriuretic peptide for predicting outcomes in acutely decompensated heart failure. Clin Cardiol. 2015; 38:106–13.
Article23. Denning GM, Ackermann LW, Barna TJ, Armstrong JG, Stoll LL, Weintraub NL, et al. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008; 29:83–92.
Article24. Beunders R, Struck J, Wu AHB, Zarbock A, Di Somma S, Mehta RL, et al. Proenkephalin (PENK) as a novel biomarker for kidney function. J App Lab Med. 2017; 2:400–12.
Article25. Ng LL, Squire IB, Jones DJL, Cao TH, Chan DCS, Sandhu JK, et al. Proenkephalin, renal dysfunction, and prognosis in patients with acute heart failure: a GREAT network study. J Am Coll Cardiol. 2017; 69:56–69.26. Caironi P, Latini R, Struck J, Hartmann O, Bergmann A, Bellato V, et al. Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem. 2018; 64:1361–9.
Article27. Rosenqvist M, Bronton K, Hartmann O, Bergmann A, Struck J, Melander O. Proenkephalin a 119–159 (penKid)–a novel biomarker for acute kidney injury in sepsis: an observational study. BMC Emerg Med. 2019; 19:75.28. Mossanen JC, Pracht J, Jansen TU, Buendgens L, Stoppe C, Goetzenich A, et al. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci. 2017; 18:1662.
Article29. Haase M, Bellomo R, Albert C, Vanpoucke G, Thomas G, Laroy W, et al. The identification of three novel biomarkers of major adverse kidney events. Biomark Med. 2014; 8:1207–17.
Article30. Ostermann M, Liu K. Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol. 2017; 31:305–14.
Article31. Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, et al. Preoperative renal risk stratification. Circulation. 1997; 95:878–84.
Article32. Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999; 14:1158–62.
Article33. Haase-Fielitz A, Haase M, Bellomo R, Calzavacca P, Spura A, Baraki H, et al. Perioperative hemodynamic instability and fluid overload are associated with increasing acute kidney injury severity and worse outcome after cardiac surgery. Blood Purif. 2017; 43:298–308.
Article34. Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. 2010; 55:2024–33.
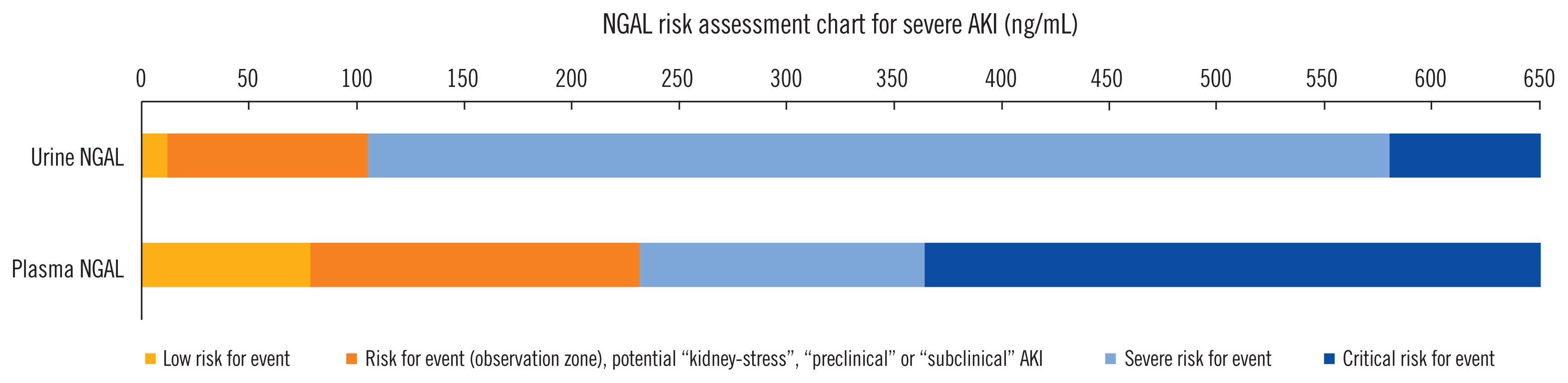
Article35. Albert C, Zapf A, Haase M, Röver C, Pickering JW, Albert A, et al. Neutrophil gelatinase-associated lipocalin measured on clinical laboratory platforms for the prediction of acute kidney injury and the associated need for dialysis therapy: a systematic review and meta-analysis. Am J Kidney Dis. 2020; https://doi.org/10.1053/j.ajkd.2020.05.015.
Article36. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. NGAL Meta-Analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009; 54:1012–24.
Article37. Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, et al. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009; 24:3349–54.
Article38. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005; 365:1231–8.
Article39. Kim SY, Jeong TD, Lee W, Chun S, Sunwoo S, Kim SB, et al. Plasma neutrophil gelatinase-associated lipocalin as a marker of tubular damage in diabetic nephropathy. Ann Lab Med. 2018; 38:524–9.
Article40. de Geus HRH, Betjes MG, Schaick Rv, Groeneveld JABJ. Plasma NGAL similarly predicts acute kidney injury in sepsis and nonsepsis. Biomark Med. 2013; 7:415–21.
Article41. Pickering JW, Endre ZH. The clinical utility of plasma neutrophil gelatinase-associated lipocalin in acute kidney injury. Blood Purif. 2013; 35:295–302.
Article42. Zhou F, Luo Q, Wang L, Han L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardiothorac Surg. 2016; 49:746–55.
Article43. Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015; 66:993–1005.
Article44. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009; 122:78–86.
Article45. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005; 115:610–21.
Article46. Ho J, Reslerova M, Gali B, Gao A, Bestland J, Rush DN, et al. Urinary hepcidin-25 and risk of acute kidney injury following cardiopulmonary bypass. Clin J Am Soc Nephrol. 2011; 6:2340–6.
Article47. Prowle JR, Ostland V, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, et al. Greater increase in urinary hepcidin predicts protection from acute kidney injury after cardiopulmonary bypass. Nephrol Dial Transplant. 2012; 27:595–602.
Article48. van Swelm RPL, Wetzels JFM, Verweij VGM, Laarakkers CMM, Pertijs JCLM, van der Wijst J, et al. Renal handling of circulating and renal-synthesized hepcidin and its protective effects against hemoglobin-mediated kidney injury. J Am Soc Nephrol. 2016; 27:2720–32.
Article49. Yang QH, Liu DW, Long Y, Liu HZ, Chai WZ, Wang XT. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infect. 2009; 58:459–64.
Article50. Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013; 17:R25.
Article51. Song Z, Ma Z, Qu K, Liu S, Niu W, Lin T. Diagnostic prediction of urinary [TIMP-2]×[IGFBP-7] for acute kidney injury: a meta-analysis exploring detection time and cutoff levels. Oncotarget. 2017; 8:100631–9.52. Food and Drug Administration. Letter to Astute Medical. 2014. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf13/den130031.pdf. Accessed July 11, 2020.53. Heung M, Ortega LM, Chawla LS, Wunderink RG, Self WH, Koyner JL, et al. Common chronic conditions do not affect performance of cell cycle arrest biomarkers for risk stratification of acute kidney injury. Nephrol Dial Transplant. 2016; 31:1633–40.
Article54. Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH 4th, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol. 2017; 312:F284–96.
Article55. Hoste E, Bihorac A, Al-Khafaji A, Ortega LM, Ostermann M, Hasse M, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med. 2020; 46:943–53.
Article56. Codorniu A, Lemasle L, Legrand M, Blet A, Mebazaa A, Gayat E. Methods used to assess the performance of biomarkers for the diagnosis of acute kidney injury: a systematic review and meta-analysis. Biomarkers. 2018; 23:766–72.
Article57. Hoste EAJ, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014; 29:2054–61.
Article58. Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, DeMuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014; 189:932–9.
Article59. Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017; 55:1074–89.
Article60. Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011; 22:1748–57.
Article61. Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011; 22:1737–47.
Article62. Di Somma S, Magrini L, De Berardinis B, Marino R, Ferri E, Moscatelli P, et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013; 17:R29.
Article63. Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the Emergency Department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012; 59:246–55.64. Hjortrup PB, Haase N, Treschow F, M⊘ller MH, Perner A. Predictive value of NGAL for use of renal replacement therapy in patients with severe sepsis. Acta Anaesthesiol Scand. 2015; 59:25–34.
Article65. Mårtensson J, Glassford NJ, Jones S, Eastwood GM, Young H, Peck L, et al. Urinary neutrophil gelatinase-associated lipocalin to hepcidin ratio as a biomarker of acute kidney injury in intensive care unit patients. Minerva Anestesiol. 2015; 81:1192–200.66. Ralib AM, Pickering JW, Shaw GM, Than MP, George PM, Endre ZH. The clinical utility window for acute kidney injury biomarkers in the critically ill. Crit Care. 2014; 18:601.
Article67. Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011; 79:1119–30.
Article68. Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014; 51:335–51.
Article69. Haase M, Kellum JA, Ronco C. Subclinical AKI–an emerging syndrome with important consequences. Nat Rev Nephrol. 2012; 8:735–9.
Article70. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204–12.71. Twerenbold R, Badertscher P, Boeddinghaus J, Nestelberger T, Wildi K, Puelacher C, et al. 0/1-hour triage algorithm for myocardial infarction in patients with renal dysfunction. Circulation. 2018; 137:436–51.72. Kellum JA, Devarajan P. What can we expect from biomarkers for acute kidney injury? Biomark Med. 2014; 8:1239–45.
Article73. Albert C, Albert A, Kube J, Bellomo R, Wettersten N, Kuppe H, et al. Urinary biomarkers may provide prognostic information for subclinical acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2018; 155:2441–52.
Article74. Joannidis M, Forni LG, Haase M, Koyner J, Shi J, Kashani K, et al. Use of cell cycle arrest biomarkers in conjunction with classical markers of acute kidney injury. Crit Care Med. 2019; 47:e820–6.
Article75. Moledina DG, Parikh CR. Phenotyping of acute kidney injury: beyond serum creatinine. Semin Nephrol. 2018; 38:3–11.
Article76. Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014; 127:94–100.
Article77. Kangasniemi OP, Biancari F, Luukkonen J, Vuorisalo S, Satta J, Pokela R, et al. Preoperative C-reactive protein is predictive of long-term outcome after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006; 29:983–5.
Article78. Xie Y, Ankawi G, Yang B, Garzotto F, Passannante A, Breglia A, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)•IGF-binding protein-7 (IGFBP-7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int. 2019; 95:1486–93.79. Albert C, Haase M, Albert A, Kropf S, Bellomo R, Westphal S, et al. Urinary biomarkers may complement the Cleveland score for prediction of adverse kidney events after cardiac surgery: a pilot study. Ann Lab Med. 2020; 40:131–41.
Article80. Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob HG, et al. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004; 50:552–8.
Article81. Katz N, Ronco C. Acute kidney stress–a useful term based on evolution in the understanding of acute kidney injury. Crit Care. 2016; 20:23.
Article82. de Geus HRH, Ronco C, Haase M, Jacob L, Lewington A, Vincent JL. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin (CSA-NGAL) score: a potential tool to monitor acute tubular damage. J Thorac Cardiovasc Surg. 2016; 151:1476–81.
Article83. Vanmassenhove J, Van Biesen W, Vanholder R, Lameire N. Subclinical AKI: ready for primetime in clinical practice? J Nephrol. 2019; 32:9–16.
Article84. Au V, Feit J, Barasch J, Sladen RN, Wagener G. Urinary neutrophil gelatinase-associated lipocalin (NGAL) distinguishes sustained from transient acute kidney injury after general surgery. Kidney Int Rep. 2016; 1:3–9.
Article85. Damman K, Valente MAE, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014; 35:455–69.
Article86. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007; 13:422–30.
Article87. Maisel AS, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, et al. Neutrophil gelatinase-associated lipocalin for acute kidney injury during acute heart failure hospitalizations: the AKINESIS study. J Am Coll Cardiol. 2016; 68:1420–31.88. Wettersten N, Horiuchi Y, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, et al. Short-term prognostic implications of serum and urine neutrophil gelatinase-associated lipocalin in acute heart failure: findings from the AKINESIS study. Eur J Heart Fail. 2020; 22:251–63.
Article89. Dupont M, Shrestha K, Singh D, Awad A, Kovach C, Scarcipino M, et al. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail. 2012; 14:597–604.
Article90. Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011; 80:405–14.
Article91. Bellomo R, Bagshaw S, Langenberg C, Ronco C. Pre-renal azotemia: a flawed paradigm in critically ill septic patients? Contrib Nephrol. 2007; 156:1–9.
Article92. Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, et al. Clinical use of the urine biomarker [TIMP-2]×[IGFBP-7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016; 68:19–28.93. Bell M, Larsson A, Venge P, Bellomo R, Mårtensson J. Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers. 2015; 2015:158658.
Article94. Delcroix G, Gillain N, Moonen M, Radermacher L, Damas F, Minon JM, et al. NGAL usefulness in the Intensive Care Unit three hours after cardiac surgery. ISRN Nephrol. 2012; 2013:865164.
Article95. Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol. 2014; 25:1063–71.
Article96. Singer E, Schrezenmeier EV, Elger A, Seelow ER, Krannich A, Luft FC, et al. Urinary NGAL-positive acute kidney injury and poor long-term outcomes in hospitalized patients. Kidney Int Rep. 2016; 1:114–24.
Article97. Koyner JL, Shaw AD, Chawla LS, Hoste EAJ, Bihorac A, Kashani K, et al. Tissue Inhibitor Metalloproteinase-2 (TIMP-2)•IGF-Binding Protein-7 (IGFBP-7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015; 26:1747–54.
Article98. Hsu CY, Chinchilli VM, Coca S, Devarajan P, Ghahramani N, Go AS, et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression: the Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) study. JAMA Intern Med. 2020; 180:402–10.99. Klein SJ, Brandtner AK, Lehner GF, Ulmer H, Bagshaw SM, Wiedermann CJ, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2018; 44:323–36.
Article100. Pickering JW, Endre ZH. Linking injury to outcome in acute kidney injury: a matter of sensitivity. PLoS One. 2013; 8:e62691.
Article101. Kim H, Hur M, Struck J, Bergmann A, Di Somma S. Proenkephalin predicts organ failure, renal replacement therapy, and mortality in patients with sepsis. Ann Lab Med. 2020; 40:466–73.
Article102. Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol. 2012; 7:1355–64.
Article103. Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008; 27:157–72.
Article104. Choi N, Rigatto C, Zappitelli M, Gao A, Christie S, Hiebert B, et al. Urinary Hepcidin-25 is elevated in patients that avoid acute kidney injury following cardiac surgery. Can J Kidney Health Dis. 2018; 5:20543581 17744224.
Article105. Levante C, Ferrari F, Manenti C, Husain-Syed F, Scarpa M, Hinna Danesi T, et al. Routine adoption of TIMP2 and IGFBP-7 biomarkers in cardiac surgery for early identification of acute kidney injury. Int J Artif Organs. 2017; 40:714–8.
Article106. Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Boerger EAS, Mc Causland FR, et al. Iron, hepcidin, and death in human AKI. J Am Soc Nephrol. 2019; 30:493–504.
Article107. Rizo-Topete LM, Rosner MH, Ronco C. Acute kidney injury risk assessment and the Nephrology Rapid Response Team. Blood Purif. 2017; 43:82–8.
Article108. Haase-Fielitz A, Albert C, Haase M. Early warning systems in acute kidney insufficiency. Nephrologe. 2017; 12:318–22.109. Albert C, Albert A, Bellomo R, Kropf S, Devarajan P, Westphal S, et al. Urinary neutrophil gelatinase-associated lipocalin-guided risk assessment for major adverse kidney events after open-heart surgery. Biomark Med. 2018; 12:975–85.
Article110. Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017; 43:1551–61.
Article111. Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg. 2018; 267:1013–20.112. Parikh A, Rizzo JA, Canetta P, Forster C, Sise M, Maarouf O, et al. Does NGAL reduce costs? A cost analysis of urine NGAL (uNGAL) & serum creatinine (sCr) for acute kidney injury (AKI) diagnosis. PLoS One. 2017; 12:e0178091.113. Meeusen JW, Rule AD, Voskoboev N, Baumann NA, Lieske JC. Performance of cystatin C– and creatinine-based estimated glomerular filtration rate equations depends on patient characteristics. Clin Chem. 2015; 61:1265–72.
Article114. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015; 24:295–300.
Article115. Luis-Lima S, Escamilla-Cabrera B, Negrín-Mena N, Estupiñán S, Delgado-Mallén P, Marrero-Miranda D, et al. Chronic kidney disease staging with cystatin C or creatinine-based formulas: flipping the coin. Nephrol Dial Transplant. 2019; 34:287–94.
Article116. Bongiovanni C, Magrini L, Salerno G, Gori CS, Cardelli P, Hur M, et al. Serum cystatin C for the diagnosis of acute kidney injury in patients admitted in the emergency department. Dis Markers. 2015; 2015:416059.
Article117. Marino R, Struck J, Hartmann O, Maisel AS, Rehfeldt M, Magrini L, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol. 2015; 28:717–24.
Article118. Hollinger A, Wittebole X, François B, Pickkers P, Antonelli M, Gayat E, et al. Proenkephalin A 119–159 (Penkid) is an early biomarker of septic acute kidney injury: the Kidney in Sepsis and Septic Shock (Kid-SSS) study. Kidney Int Rep. 2018; 3:1424–33.
Article119. Doemming S, Simon TP, Humbs A, Martin L, Bruells C, Hartmann O, et al. Pro-enkephalin in plasma of surgical icu-patients with sepsis - a pilot study. Intensive Care Med Exp. 2015; 3(S1):A256.
Article120. Nadim MK, Forni LG, Bihorac A, Hobson C, Koyner JL, Shaw A, et al. Cardiac and vascular surgery–associated acute kidney injury: the 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group. J Am Heart Assoc. 2018; 7:e008834.
Article121. Boeddinghaus J, Nestelberger T, Twerenbold R, Wildi K, Badertscher P, Cupa J, et al. Direct comparison of 4 very early rule-out strategies for acute myocardial infarction using high-sensitivity cardiac troponin I. Circulation. 2017; 135:1597–611.122. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002; 347:161–7.
Article123. Pufulete M, Maishman R, Dabner L, Higgins JPT, Rogers CA, Dayer M, et al. B-type natriuretic peptide-guided therapy for heart failure (HF): a systematic review and meta-analysis of individual participant data (IPD) and aggregate data. Syst Rev. 2018; 7:112.
Article124. de Grooth HJ, Parienti JJ, Schetz M. AKI biomarkers are poor discriminants for subsequent need for renal replacement therapy, but do not disqualify them yet. Intensive Care Med. 2018; 44:1156–8.
Article125. Devarajan P. NGAL for the detection of acute kidney injury in the emergency room. Biomark Med. 2014; 8:217–9.
Article126. Devarajan P, Murray P. Biomarkers in acute kidney injury: are we ready for prime time? Nephron Clin Pract. 2014; 127:176–9.
Article127. Doi K, Yuen PST, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009; 20:1217–21.
Article128. Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009; 14:423–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepcidin-25 as a Novel Kidney Biomarker for Cardiac Surgery-Associated Acute Kidney Injury
- Experimental evidence that preexisting chronic kidney disease is a risk factor for acute kidney injury
- Hepcidin and Neutrophil Gelatinase-Associated Lipocalin as a Biomarker for Acute Kidney Injury Linked Iron Metabolism
- A Novel Biomarker for the Acute Kidney Injury in the Cirrhotic Patients
- Acute kidney injury in cardiac surgery