Ann Lab Med.
2021 Jul;41(4):414-418. 10.3343/alm.2021.41.4.414.
A Stabilizing Agent, PCA/DTPA, Improves Plasma Storage Life for the Chromsystems Vitamin C Assay up to Six Months
- Affiliations
-
- 1School of Health and Biomedical Sciences, RMIT University, Bundoora, Victoria, Australia
- 2Royal College of Pathologists Quality Assurance Programs Vitamins Advisory Committee, Sydney, New South Wales, Australia
- 3Dubbo Hospital, Western NSW Local Health District, Dubbo, New South Wales, Australia
- 4Department of Critical Care, The University of Melbourne, Melbourne, Australia
- 5Department of Intensive Care, Royal Melbourne Hospital, Parkville, Victoria, Australia
- 6Department of Intensive Care, Austin Health, Heidelberg, Australia
- 7Victorian Clinical Genetics Services, Murdoch Children’s Research Institute, Parkville, Victoria, Australia
- 8Department of Paediatrics, University of Melbourne, Melbourne, VIC, Australia
- KMID: 2512722
- DOI: http://doi.org/10.3343/alm.2021.41.4.414
Abstract
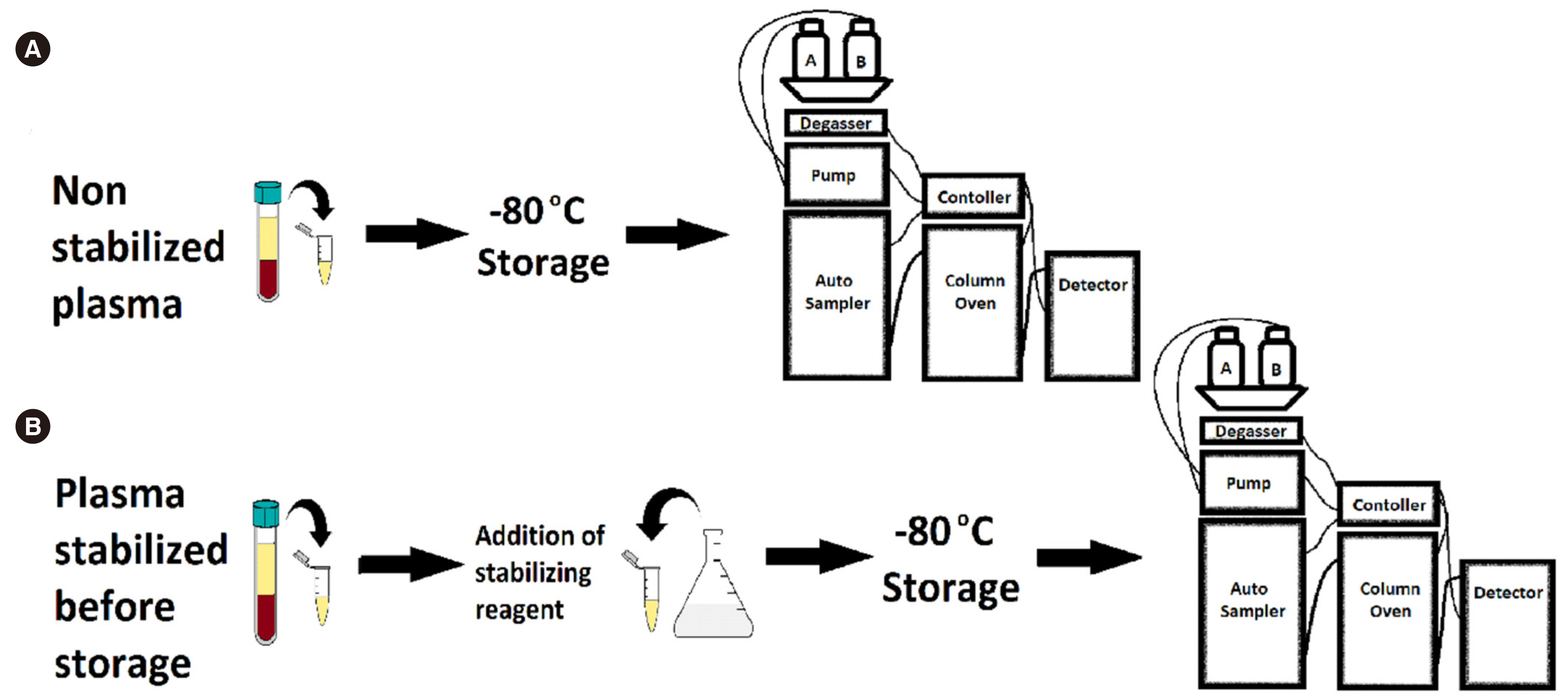
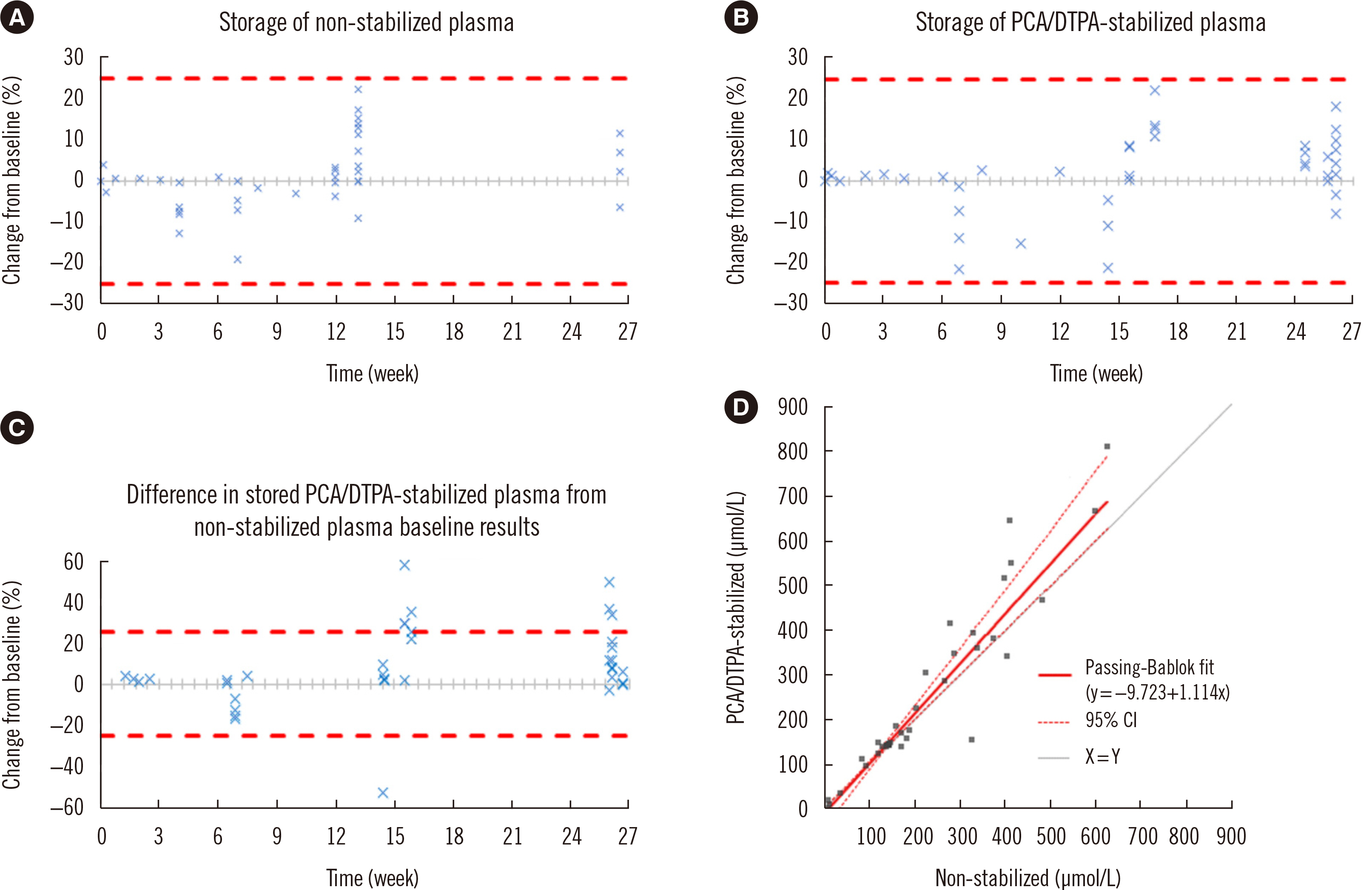
- The commonly used Chromsystems vitamin C (ascorbate) assay (Munich, Germany) has a sample storage life of five days at –20°C. Stabilizing agents have been successfully used to increase longevity; however, their suitability with this commercial assay is unclear. We investigated the compatibility of a stabilizing agent, perchloric acid/diethylenetriaminepentaacetic acid (PCA/DTPA), with the Chromsystems assay. Plasma was stored at –80°C, with or without PCA/DTPA. Storage up to six months was assessed through baseline and repeat analyses, stability was assessed by comparing paired non-stabilized and PCA/ DTPA-stabilized plasma, and performance was assessed using allowable performance specifications of an external quality assurance program. Ascorbate concentration was significantly lower in non-stabilized plasma than in paired PCA/DTPA-stabilized plasma, with a proportional difference of 11% (P = 0.01). All storage analysis results were within the allowable performance specifications. Storage at –80°C prevented plasma ascorbate oxidation; however, substantial oxidation occurred during sample processing. In conclusion, PCA/DTPA significantly reduces ascorbate oxidation, and PCA/DTPA-stabilized ascorbate plasma is compatible with the Chromsystems assay and stable for up to six months, when stored at –80°C.
Figure
Reference
-
1. Collie JTB, Greaves RF, Jones OAH, Eastwood GM, Bellomo R. 2020; Vitamin C measurement in critical illness: challenges, methodologies and quality improvements. Clin Chem Lab Med. 58:460–70. DOI: 10.1515/cclm-2019-0912. PMID: 31829967.
Article2. RCPA Quality Assurance Programs. Vitamin C End of Cycle 41 Report. https://myqap.rcpaqap.com.au/reports. Updated on Dec 2019.3. Salminen I, Alfthan G. 2008; Plasma ascorbic acid preparation and storage for epidemiological studies using TCA precipitation. Clin Biochem. 41:723–7. DOI: 10.1016/j.clinbiochem.2007.01.026. PMID: 18371942.
Article4. Karlsen A, Blomhoff R, Gundersen TE. 2007; Stability of whole blood and plasma ascorbic acid. Eur J Clin Nutr. 61:1233–6. DOI: 10.1038/sj.ejcn.1602655. PMID: 17299479.
Article5. Pullar JM, Bayer S, Carr AC. 2018; Appropriate handling, processing and analysis of blood samples is essential to avoid oxidation of vitamin C to dehydroascorbic acid in clinical samples. Antioxidants. 7:29. DOI: 10.3390/antiox7020029. PMID: 29439480. PMCID: PMC5836019.6. Lykkesfeldt J. 2007; Ascorbate and dehydroascorbic acid as reliable biomarkers of oxidative stress: analytical reproducibility and long-term stability of plasma samples subjected to acidic deproteinization. Cancer Epidemiol Biomarkers Prev. 16:2513–6. DOI: 10.1158/1055-9965.EPI-07-0639. PMID: 18006947.
Article7. Rossi B, Tittone F, Palleschi S. 2016; Setup and validation of a convenient sampling procedure to promptly and effectively stabilize vitamin C in blood and plasma specimens stored at routine temperatures. Anal Bioanal Chem. 408:4723–31. DOI: 10.1007/s00216-016-9560-6. PMID: 27113458.
Article8. Jenab M, Bingham S, Ferrari P, Friesen MD, Al-Delaimy WK, Luben R, et al. 2005; Long-term cryoconservation and stability of vitamin C in serum samples of the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 14:1837–40. DOI: 10.1158/1055-9965.EPI-05-0061. PMID: 16030126.
Article9. Fujii T, Udy AA, Deane AM, Luethi N, Bailey M, Eastwood GM, et al. 2019; Vitamin C, Hydrocortisone and Thiamine in Patients with Septic Shock (VITAMINS) trial: study protocol and statistical analysis plan. Crit Care Resusc. 21:119–25.10. Bobrowicz E, Naskalski JW, Siedlecki A. 2001; Preanalytical factors in human plasma ascorbate assay. Clin Chim Acta. 314:237–9. DOI: 10.1016/S0009-8981(01)00577-0.
Article11. Lykkesfeldt J. 2012; Ascorbate and dehydroascorbic acid as biomarkers of oxidative stress: validity of clinical data depends on Vacutainer system used. Nutr Res. 32:66–9. DOI: 10.1016/j.nutres.2011.11.005. PMID: 22260866.
Article12. Talwar DK, Azharuddin MK, Williamson C, Teoh YP, McMillan DC, St J O'Reilly D. 2005; Biological variation of vitamins in blood of healthy individuals. Clin Chem. 51:2145–50. DOI: 10.1373/clinchem.2005.056374. PMID: 16155091.
Article13. Ching SYL, Prins AW, Beilby JP. 2002; Stability of ascorbic acid in serum and plasma prior to analysis. Ann Clin Biochem. 39:518–20. DOI: 10.1258/000456302320314566. PMID: 12227861.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Analysis of Vitamin C Concentration in Organs of Gulo-/- Mice Upon Vitamin C Withdrawal
- Plasma Oligomeric Beta Amyloid in Alzheimer's Disease with History of Agent Orange Exposure
- Altered Responses of IL-6 and IL-10 by the Ketorolac as an Adjunct to Patient-Controlled Morphine after bdominal Hysterectomy
- Comparison of DMSA scan and DTPA scan for evaluation of relative renal function in pediatric hydronephrosis
- Evaluation of Renal Function before and/or after Operation Using 99mTc-DTPA Renal Scan