Healthc Inform Res.
2021 Jan;27(1):19-28. 10.4258/hir.2021.27.1.19.
Effectiveness of Transfer Learning for Deep Learning-Based Electrocardiogram Analysis
- Affiliations
-
- 1Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea
- 2Department of Biomedical Sciences, Graduate School of Medicine, Ajou University, Suwon, Korea
- KMID: 2512621
- DOI: http://doi.org/10.4258/hir.2021.27.1.19
Abstract
Objectives
Many deep learning-based predictive models evaluate the waveforms of electrocardiograms (ECGs). Because deep learning-based models are data-driven, large and labeled biosignal datasets are required. Most individual researchers find it difficult to collect adequate training data. We suggest that transfer learning can be used to solve this problem and increase the effectiveness of biosignal analysis.
Methods
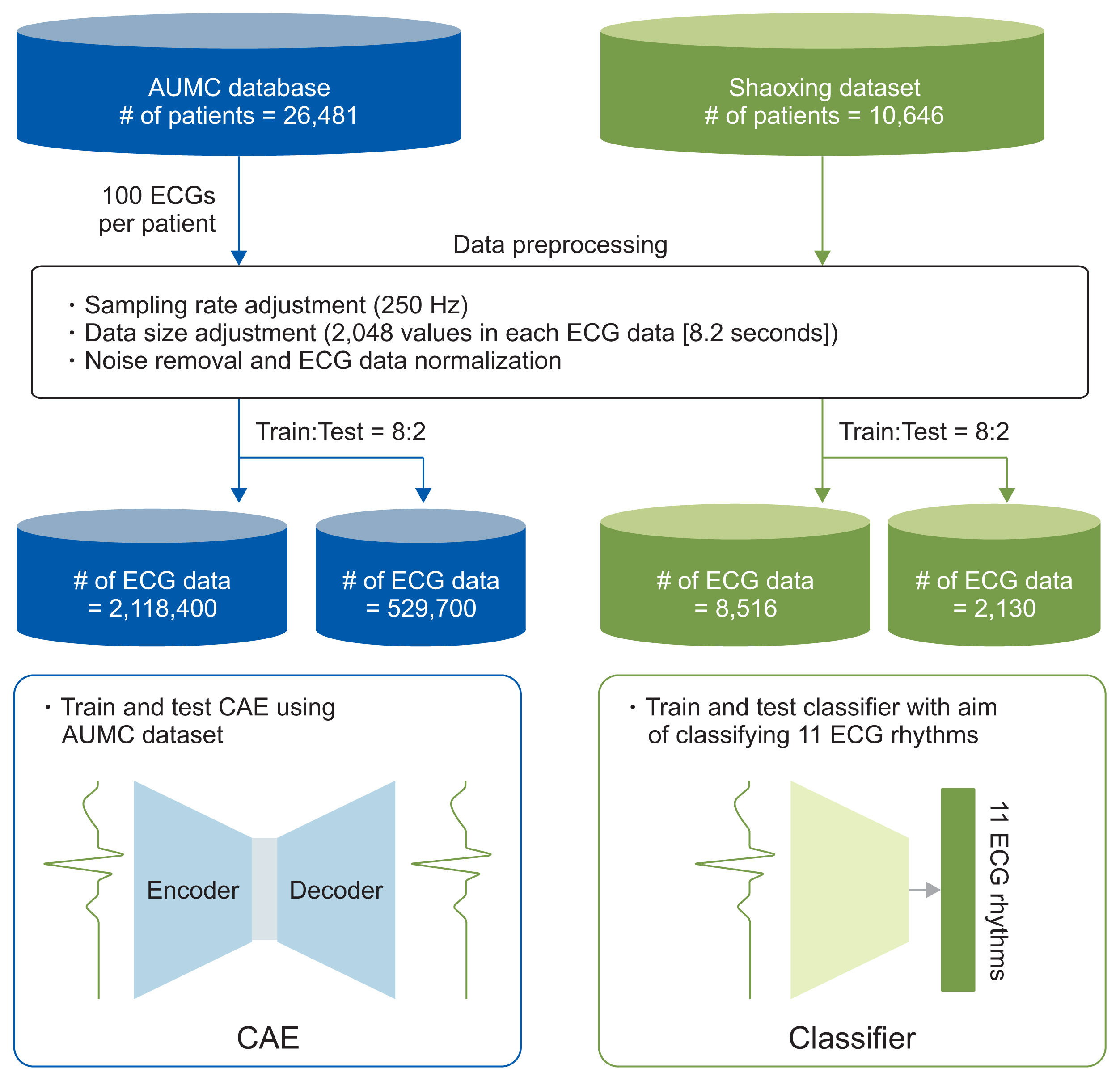
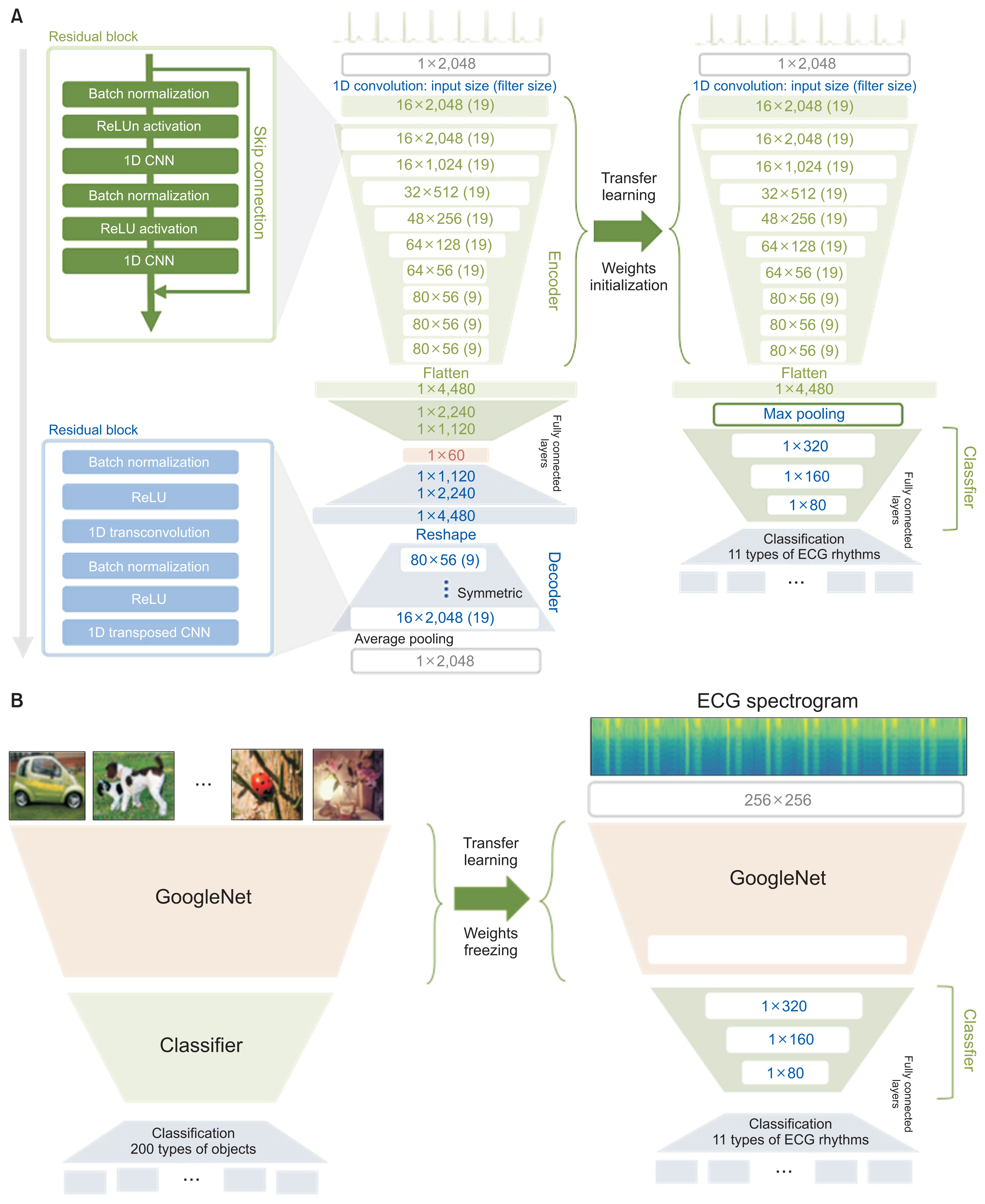
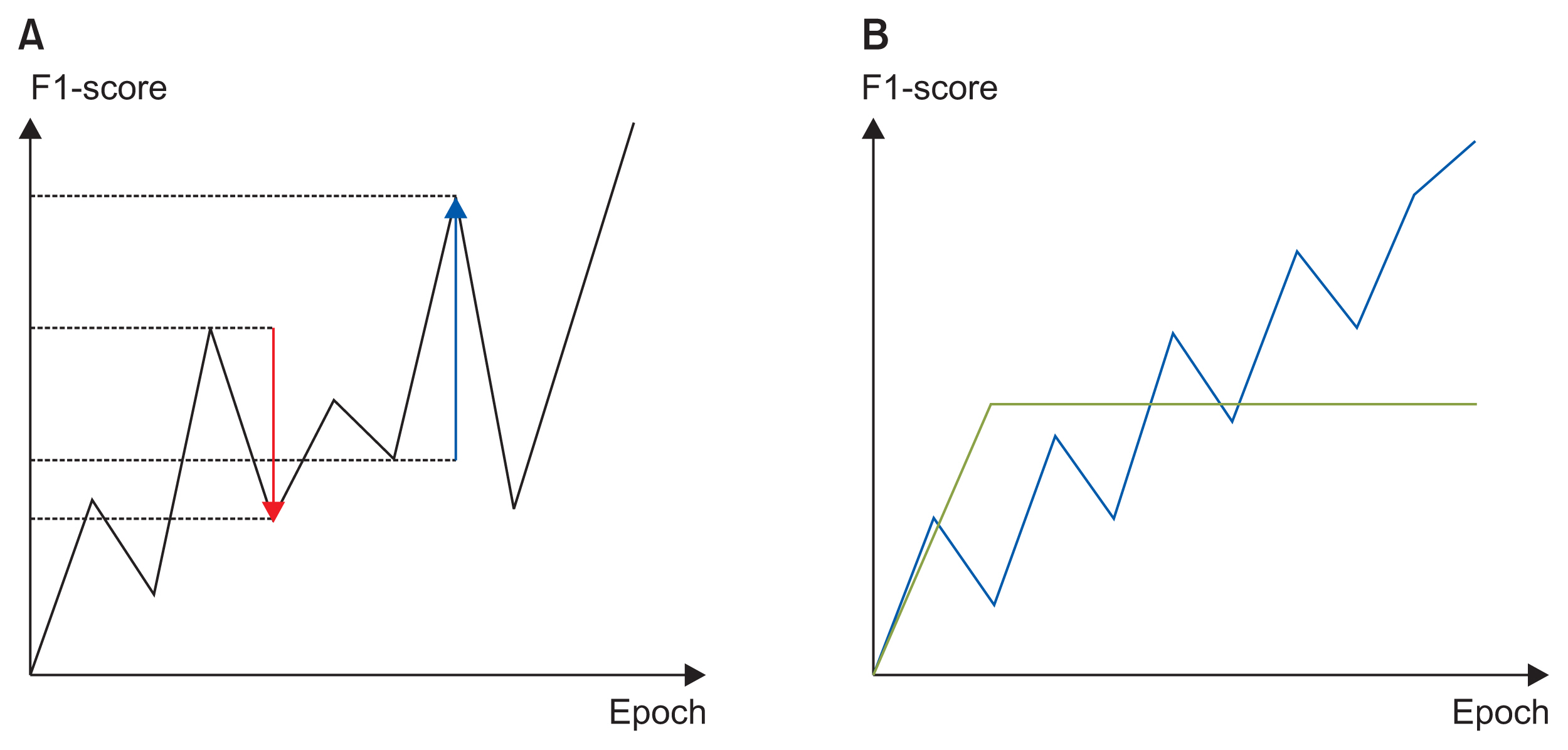
We applied the weights of a pretrained model to another model that performed a different task (i.e., transfer learning). We used 2,648,100 unlabeled 8.2-second-long samples of ECG II data to pretrain a convolutional autoencoder (CAE) and employed the CAE to classify 12 ECG rhythms within a dataset, which had 10,646 10-second-long 12-lead ECGs with 11 rhythm labels. We split the datasets into training and test datasets in an 8:2 ratio. To confirm that transfer learning was effective, we evaluated the performance of the classifier after the proposed transfer learning, random initialization, and two-dimensional transfer learning as the size of the training dataset was reduced. All experiments were repeated 10 times using a bootstrapping method. The CAE performance was evaluated by calculating the mean squared errors (MSEs) and that of the ECG rhythm classifier by deriving F1-scores.
Results
The MSE of the CAE was 626.583. The mean F1-scores of the classifiers after bootstrapping of 100%, 50%, and 25% of the training dataset were 0.857, 0.843, and 0.835, respectively, when the proposed transfer learning was applied and 0.843, 0.831, and 0.543, respectively, after random initialization was applied.
Conclusions
Transfer learning effectively overcomes the data shortages that can compromise ECG domain analysis by deep learning.
Figure
Reference
-
References
1. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019; 394:861–7.
Article2. van Ravenswaaij-Arts CM, Kollee LA, Hopman JC, Stoelinga GB, van Geijn HP. Heart rate variability. Ann Intern Med. 1993; 118:436–47.
Article3. Al-Zaiti S, Besomi L, Bouzid Z, Faramand Z, Frisch S, Martin-Gill C, et al. Machine learning-based prediction of acute coronary syndrome using only the pre-hospital 12-lead electrocardiogram. Nat Commun. 2020; 11:3966.
Article4. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019; 25:70–4.
Article5. Isakadze N, Martin SS. How useful is the smartwatch ECG? Trends Cardiovasc Med. 2020; 30:442–8.
Article6. Pan SJ, Yang Q. A survey on transfer learning. IEEE Trans Knowl Data Eng. 2009; 22:1345–59.
Article7. Zhao W. Research on the deep learning of the small sample data based on transfer learning. AIP Conf Proc. 2017; 1864:020018.
Article8. Raghu M, Zhang C, Kleinberg J, Bengio S. Transfusion: understanding transfer learning for medical imaging [Internet]. Ithaca (NY): arXiv.org;2019. [cited at 2021 Jan 4]. Available from: https://arxiv.org/abs/1902.07208.9. Ranti D, Hanss K, Zhao S, Arvind V, Titano J, Costa A, et al. The utility of general domain transfer learning for medical language tasks [Internet]. Ithaca (NY): arXiv.org;2020. [cited at 2021 Jan 4]. Available from: https://arxiv.org/abs/2002.06670.10. Van Steenkiste G, van Loon G, Crevecoeur G. Transfer learning in ECG classification from human to horse using a novel parallel neural network architecture. Sci Rep. 2020; 10:186.
Article11. Tadesse GA, Zhu T, Liu Y, Zhou Y, Chen J, Tian M, et al. Cardiovascular disease diagnosis using cross-domain transfer learning. Annu Int Conf IEEE Eng Med Biol Soc. 2019; 2019:4262–5.
Article12. Salem M, Taheri S, Yuan JS. ECG arrhythmia classification using transfer learning from 2-dimensional deep CNN features. In : Proceedings of 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS); 2018 Oct 17–19; Cleveland, OH.
Article13. Abdelazez M, Rajan S, Chan AD. Transfer learning for detection of atrial fibrillation in deterministic compressive sensed ECG. Annu Int Conf IEEE Eng Med Biol Soc. 2020; 2020:5398–401.
Article14. Wen L, Gao L, Li X. A new deep transfer learning based on sparse auto-encoder for fault diagnosis. IEEE Trans Syst Man Cybern Syst. 2017; 49:136–44.
Article15. Wang C, Elazab A, Jia F, Wu J, Hu Q. Automated chest screening based on a hybrid model of transfer learning and convolutional sparse denoising autoencoder. Biomed Eng Online. 2018; 17:63.
Article16. Eduardo A, Aidos H, Fred A. ECG-based biometrics using a deep autoencoder for feature learning-an empirical study on transferability. In : Proceedings of the 6th International Conference on Pattern Recognition Applications and Methods; 2017 Feb 24–26; Porto, Portugal. p. 463–70.
Article17. Yoon D, Lee S, Kim TY, Ko J, Chung WY, Park RW. System for collecting biosignal data from multiple patient monitoring systems. Healthc Inform Res. 2017; 23:333–7.
Article18. Zheng J, Zhang J, Danioko S, Yao H, Guo H, Rakovski C. A 12-lead electrocardiogram database for arrhythmia research covering more than 10,000 patients. Sci Data. 2020; 7:48.
Article19. Baldi P. Autoencoders, unsupervised learning, and deep architectures. In : Proceedings of the International Conference on Machine Learning (ICML): Unsupervised and Transfer Learning; 2012 Jun 26–Jul 1; Edinburgh, Scotland. p. 37–49.20. Liu W, Wang Z, Liu X, Zeng N, Liu Y, Alsaadi FE. A survey of deep neural network architectures and their applications. Neurocomputing. 2017; 234:11–26.
Article21. Qin Z, Yu F, Liu C, Chen X. How convolutional neural network see the world-A survey of convolutional neural network visualization methods [Internet]. Ithaca (NY): arXiv.org;2018. [cited at 2021 Jan 4]. Available from: https://arxiv.org/abs/1804.11191.22. Deng J, Dong W, Socher R, Li LJ, Li K, Li FF. ImageNet: a large-scale hierarchical image database. In : Proceedings of 2009 IEEE Conference on Computer Vision and Pattern Recognition; 2009 Jun 20–25; Miami, FL. p. 248–55.
Article23. Szegedy C, Liu W, Jia Y, Sermanet P, Reed S, Anguelov D, et al. Going deeper with convolutions. In : Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; 2015 Jun 7–12; Boston, MA. p. 1–9.
Article24. DiCiccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci. 1996; 11:189–212.
Article25. Kingma DP, Ba J. Adam: a method for stochastic optimization [Internet]. Ithaca (NY): arXiv.org;2014. [cited at 2021 Jan 4]. Available from: https://arxiv.org/abs/1412.6980.26. Hussain M, Bird JJ, Faria DR. A study on CNN transfer learning for image classification. Lotfi A, Bouchachia H, Gegov A, Langensiepen C, McGinnity M, editors. Advances in computational intelligence systems. Cham, Switzerland: Springer;2018. p. 191–202.
Article27. Huh M, Agrawal P, Efros AA. What makes ImageNet good for transfer learning? [Internet]. Ithaca (NY): arXiv. org;2016. [cited at 2021 Jan 4]. Available from: https://arxiv.org/abs/1608.08614.28. Porumb M, Stranges S, Pescape A, Pecchia L. Precision medicine and artificial intelligence: a pilot study on deep learning for hypoglycemic events detection based on ECG. Sci Rep. 2020; 10:170.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep Learning in Dental Radiographic Imaging
- Current status of deep learning applications in abdominal ultrasonography
- Deep Learning in MR Motion Correction:a Brief Review and a New Motion Simulation Tool (view2Dmotion)
- Deep Learning in Nuclear Medicine and Molecular Imaging: Current Perspectives and Future Directions
- A Review on Usage and Effectiveness of e-Learning in Medical Education