Korean Circ J.
2021 Feb;51(2):157-170. 10.4070/kcj.2020.0255.
High Level of Real Urban Air Pollution Promotes Cardiac Arrhythmia in Healthy Mice
- Affiliations
-
- 1Division of Cardiology, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Cardiology, School of Medicine, Ewha Womans University, Seoul, Korea
- 3Department of Mechanical Engineering, Dankook University, Yongin, Korea
- 4Department of Otorhinolaryngology, Head and Neck Surgery, Inha University College of Medicine, Incheon, Korea
- 5Department of Preventive Medicine, Yonsei University College of Medicine, Yonsei University, Seoul, Korea
- 6Department of Cardiology, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 7The Division of Cardiology, Saint Carollo Hospital, Suncheon, Korea
- KMID: 2512454
- DOI: http://doi.org/10.4070/kcj.2020.0255
Abstract
- Background and Objectives
Ambient particulate matter (PM) in real urban air pollution (RUA) is an environmental health risk factor associated with increased cardiac events. This study investigated the threshold level to induce arrhythmia, as well as arrhythmogenic mechanism of RUA that mainly consisted of PM <2.5 μm in aerodynamic diameter close to ultrafine particles.
Methods
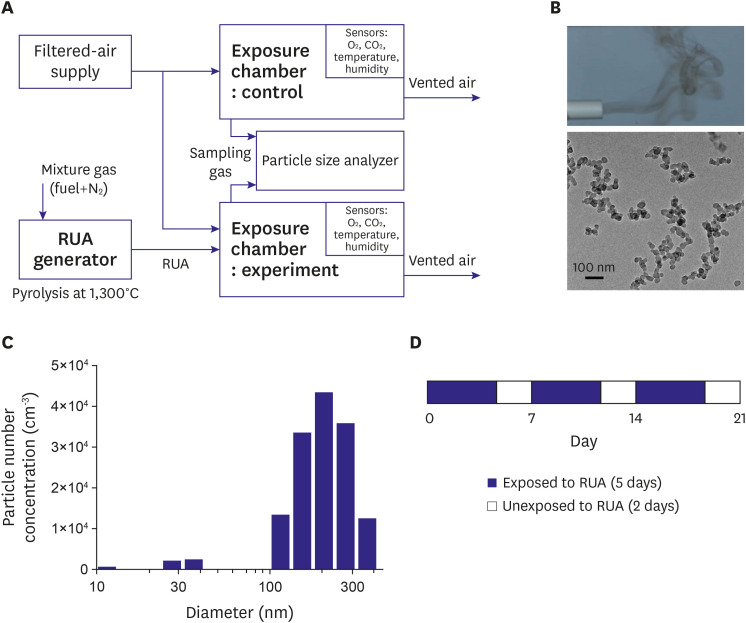
RUA was artificially produced by a lately developed pyrolysis based RUA generator.C57BL/6 mice were divided into 4 groups: a control group (control, n=12) and three groups with exposure to RUA with the concentration of 200 µg/㎥ (n=12), 400 µg/㎥ (n=12), and 800 µg/㎥ (n=12). Mice were exposed to RUA at each concentration for 8 hr/day and 5 day/week to mimic ordinary human activity during 3 weeks.
Results
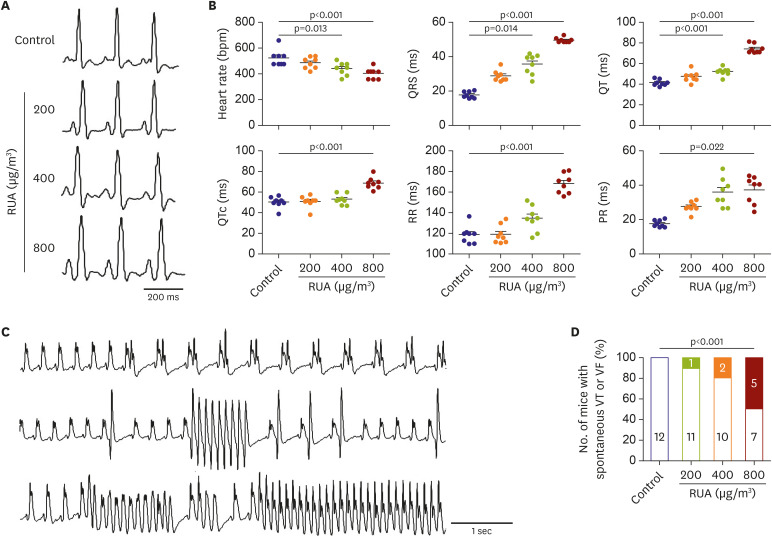
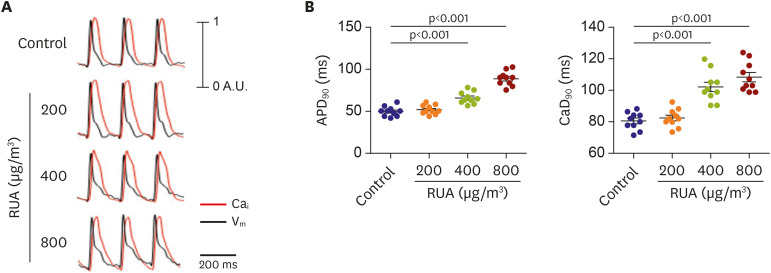
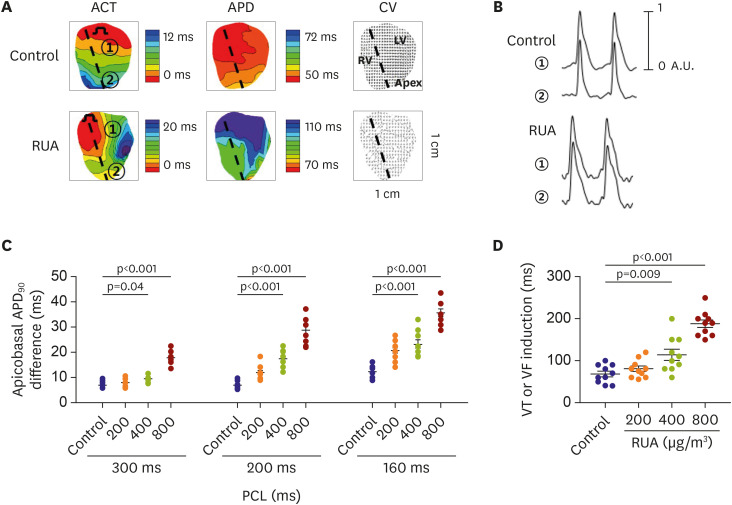
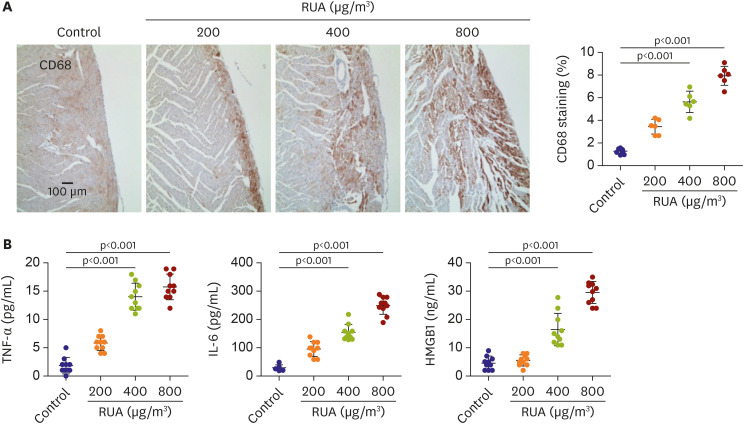
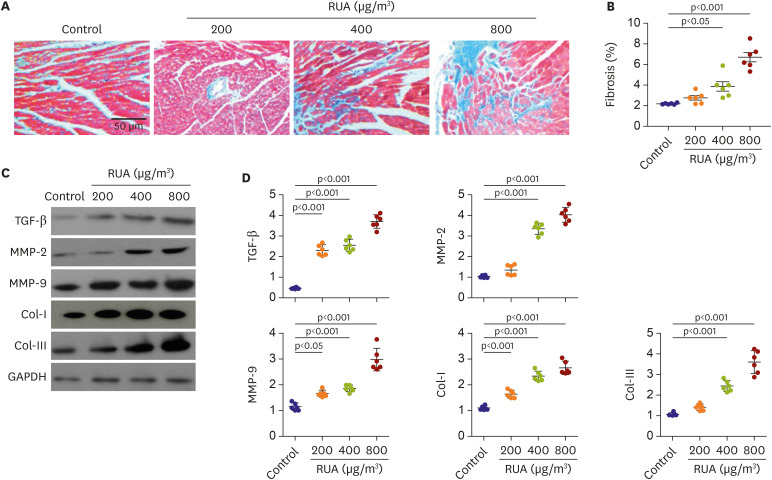
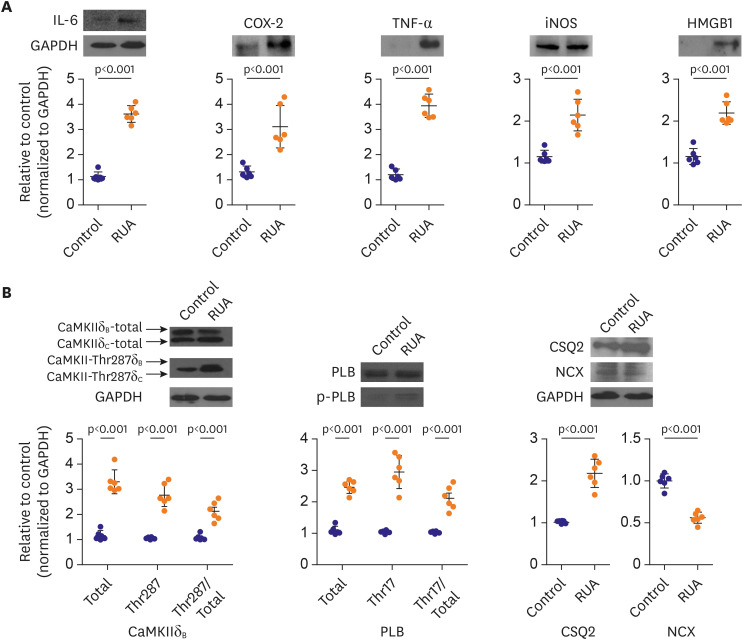
The QRS and QTc intervals, as well as intracellular Ca2+ duration, apicobasal action potential duration (APD) gradient, fibrosis, and inflammation of left ventricle of mouse hearts were increased dose-dependently with the increase of RUA concentration, and significantly increased at RUA concentration of 400 µg/㎥ compared to control (all p<0.001). In mice exposed to RUA concentration of 800 µg/㎥ , spontaneous ventricular arrhythmia was observed in 42%, with significant increase of inflammatory markers, phosphorylated Ca2+ /calmodulindependent protein kinase II (CaMKII), and phospholamban (PLB) compared to control.
Conclusions
RUA could induce electrophysiological changes such as APD and QT prolongation, fibrosis, and inflammation dose-dependently, with significant increase of ventricular arrhythmia at the concentration of 400 µg/㎥ . RUA concentration of 800 µg/㎥ increased phosphorylation of CaMKII and PLB.
Keyword
Figure
Cited by 1 articles
-
Fine Particulate Matter: a Threat to the Heart Rhythm
Jinhee Ahn
Korean Circ J. 2021;51(2):171-173. doi: 10.4070/kcj.2020.0501.
Reference
-
1. Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001; 103:2810–2815. PMID: 11401937.
Article2. Pope CA 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004; 109:71–77. PMID: 14676145.3. Kim IS, Sohn J, Lee SJ, et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implantable cardioverter defibrillators: Vulnerable patients to air pollution. Int J Cardiol. 2017; 240:214–220. PMID: 28392078.
Article4. Sohn J, You SC, Cho J, Choi YJ, Joung B, Kim C. Susceptibility to ambient particulate matter on emergency care utilization for ischemic heart disease in Seoul, Korea. Environ Sci Pollut Res Int. 2016; 23:19432–19439. PMID: 27380182.
Article5. Tobias HJ, Beving DE, Ziemann PJ, et al. Chemical analysis of diesel engine nanoparticles using a nano-DMA/thermal desorption particle beam mass spectrometer. Environ Sci Technol. 2001; 35:2233–2243. PMID: 11414024.
Article6. Huang W, Zhu T, Pan X, et al. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am J Epidemiol. 2012; 176:117–126. PMID: 22763390.
Article7. Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001; 12:355–357. PMID: 11337606.
Article8. Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005; 113:670–674. PMID: 15929887.
Article9. Ljungman PL, Berglind N, Holmgren C, et al. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008; 29:2894–2901. PMID: 19004842.
Article10. Cozzi E, Hazarika S, Stallings HW 3rd, et al. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2006; 291:H894–903. PMID: 16582015.
Article11. Zhang R, Khoo MS, Wu Y, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005; 11:409–417. PMID: 15793582.12. Erickson JR, Joiner ML, Guan X, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008; 133:462–474. PMID: 18455987.
Article13. Kim JB, Kim C, Choi E, et al. Particulate air pollution induces arrhythmia via oxidative stress and calcium calmodulin kinase II activation. Toxicol Appl Pharmacol. 2012; 259:66–73. PMID: 22197715.
Article14. Park H, Park S, Jeon H, et al. Alpha B-crystallin prevents the arrhythmogenic effects of particulate matter isolated from ambient air by attenuating oxidative stress. Toxicol Appl Pharmacol. 2013; 266:267–275. PMID: 23153557.
Article15. Cho SL, Lee W, Park S. Synthesis of primary-particle-size-tuned soot particles by controlled pyrolysis of hydrocarbon fuels. Energy Fuels. 2016; 30:6614–6619.
Article16. Park H, Park H, Hwang HJ, et al. Alpha B-crystallin prevents ventricular arrhythmia by attenuating inflammation and oxidative stress in rat with autoimmune myocarditis. Int J Cardiol. 2015; 182:399–402. PMID: 25596465.
Article17. Park H, Park H, Mun D, et al. Sympathetic nerve blocks promote anti-inflammatory response by activating the JAK2-STAT3-mediated signaling cascade in rat myocarditis models: a novel mechanism with clinical implications. Heart Rhythm. 2018; 15:770–779. PMID: 28963014.
Article18. Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006; 56:709–742. PMID: 16805397.
Article19. Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008; 52:719–726. PMID: 18718418.20. Antzelevitch C. Ionic, molecular, and cellular bases of QT-interval prolongation and torsade de pointes. Europace. 2007; 9(Suppl 4):iv4–iv15. PMID: 17766323.
Article21. Sivagangabalan G, Spears D, Masse S, et al. The effect of air pollution on spatial dispersion of myocardial repolarization in healthy human volunteers. J Am Coll Cardiol. 2011; 57:198–206. PMID: 21211691.
Article22. Silbajoris R, Ghio AJ, Samet JM, Jaskot R, Dreher KL, Brighton LE. In vivo and in vitro correlation of pulmonary MAP kinase activation following metallic exposure. Inhal Toxicol. 2000; 12:453–468. PMID: 10880139.23. Lucking AJ, Lundback M, Mills NL, et al. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008; 29:3043–3051. PMID: 18952612.
Article24. Törnqvist H, Mills NL, Gonzalez M, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007; 176:395–400. PMID: 17446340.
Article25. Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J Biol Chem. 2007; 282:10833–10839. PMID: 17296607.26. Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009; 104:79–86. PMID: 19038865.27. Nemmar A, Hoet PH, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002; 105:411–414. PMID: 11815420.
Article