Lab Med Online.
2020 Apr;10(2):160-164. 10.3343/lmo.2020.10.2.160.
Evaluating the Diagnostic Performance of Two Rapid Influenza Diagnostic Tests
- Affiliations
-
- 1Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2512247
- DOI: http://doi.org/10.3343/lmo.2020.10.2.160
Abstract
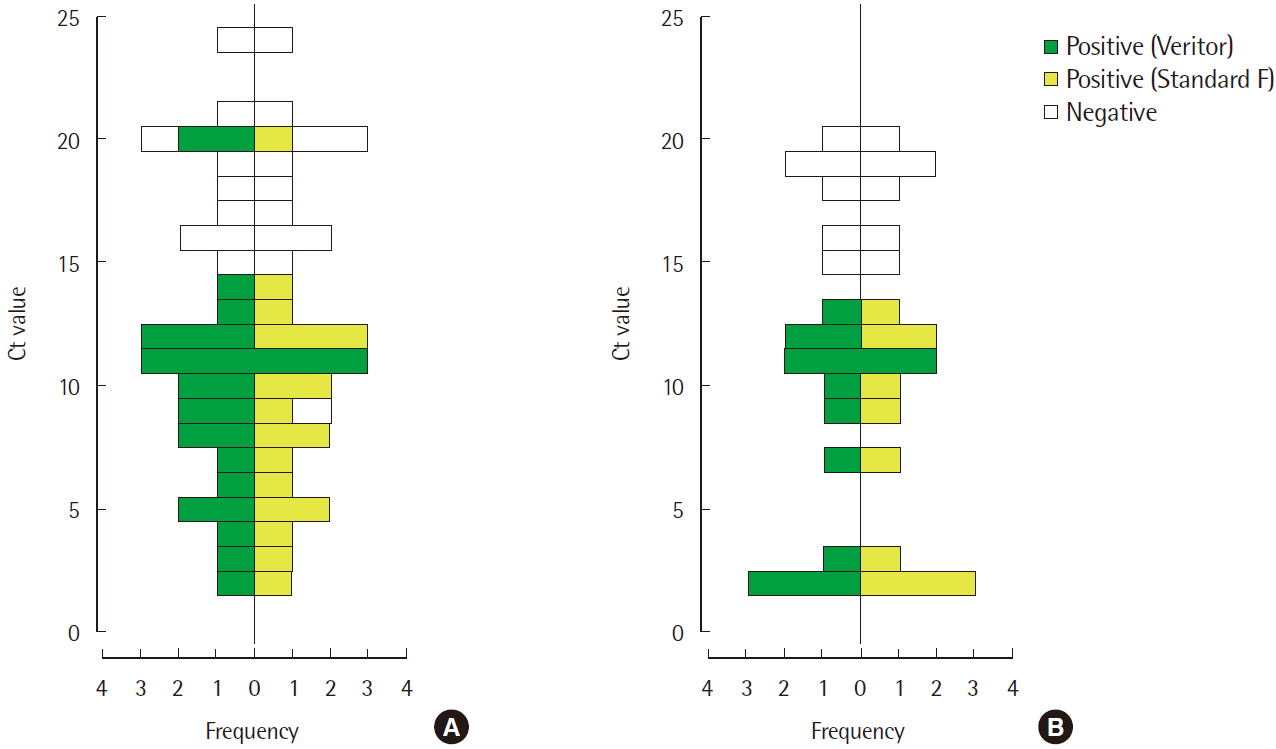
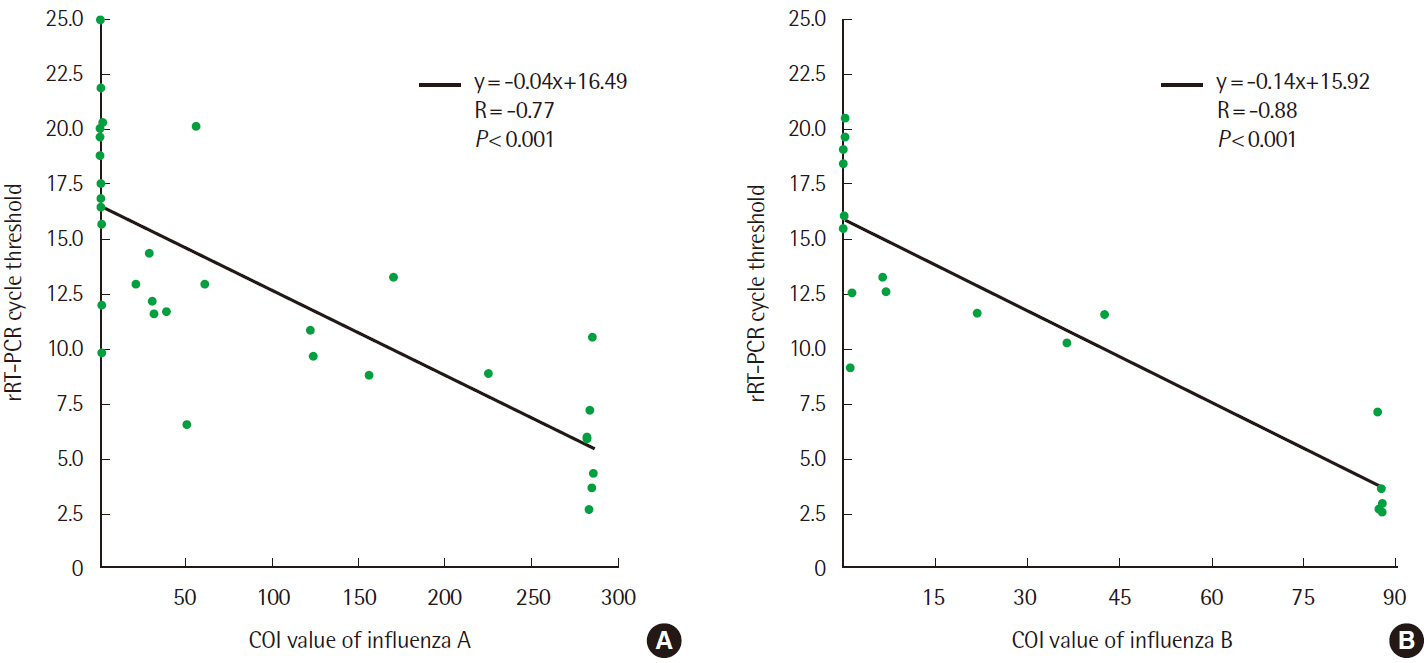
- Rapid influenza diagnostic test (RIDT) is widely used for the diagnosis of influenza owing to its simplicity and convenience of use. This study aimed to evaluate the performance of a new RIDT, SD Standard F influenza A/B FIA (SD Biosensor, Inc., Korea) (Standard F) and compare its performance with BD Veritor Flu A+B (Veritor), using the results of real-time reverse transcription PCR (rRT-PCR) analysis as the standard for reference. On comparing the results obtained from both the RIDTs and rRT-PCR qualitatively, it was found that the Veritor and Standard F assays have the sensitivity of 65.6% (21/32) and 71.9% (23/32), respectively, for the detection of influenza A with a specificity of 100.0% (68/68). Additionally, both the assays demonstrated a sensitivity of 66.7% (12/18) and specificity of 100.0% (68/68) for the detection of influenza B. The cutoff index (COI) value of the fluorescence color intensity from the Standard F assay, displayed on the device along with the qualitative results, indicated a negative correlation with the Ct value from rRT-PCR for both influenza A and B (P<0.001). The sensitivity of the new RIDT for the detection of influenza was comparable with that of the Veritor assay and the new RIDT could be used as a substitute for existing RIDTs by providing additional information to predict the approximate viral burden of influenza.
Keyword
Figure
Reference
-
1. Nicholson KG, Wood JM, Zambon M. 2003; Influenza. Lancet. 362:1733–45. DOI: 10.1016/S0140-6736(03)14854-4. PMID: 14643124. PMCID: PMC7112395.
Article2. Merckx J, Wali R, Schiller I, Caya C, Gore GC, Chartrand C, et al. 2017; Diagnostic accuracy of novel and traditional rapid tests for influenza infec-tion compared with reverse transcriptase polymerase chain reaction: A systematic review and metaanalysis. Ann Intern Med. 167:394–409. DOI: 10.7326/M17-0848. PMID: 28869986.3. Centers for disease control and prevention (CDC). Information for clinicians on influenza virus testing. https://www.cdc.gov/flu/profes-sionals/diagnosis/index.htm. Updated on Feb. 2018.4. Tsushima Y, Uno N, Sasaki D, Morinaga Y, Hasegawa H, Yanagihara K. 2015; Quantitative RT-PCR evaluation of a rapid influenza antigen test for efficient diagnosis of influenza virus infection. J Virol Methods. 212:76–9. DOI: 10.1016/j.jviromet.2014.10.019. PMID: 25449113.
Article5. Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. 2012; Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 156:500–11. DOI: 10.7326/0003-4819-156-7-201204030-00403. PMID: 22371850.6. World Health Organization. WHO recommendations on the use of rapid testing for influenza diagnosis. https://www.who.int/influenza/resources/documents/rapid_testing/en/. Updated on Jul. 2005.7. Park S, Lee M, Chung HS. 2015; Evaluation of the performance of a new chro-matographic assay BD Veritor™ System for rapid detection of influenza A & B. Ann Clin Microbiol. 18:27–32. DOI: 10.5145/ACM.2015.18.1.27.8. Yeo SJ, Bao DT, Seo GE, Bui CT, Kim DTH, Anh NTV, et al. 2017; Improvement of a rapid diagnostic application of monoclonal antibodies against avian influenza H7 subtype virus using Europium nanoparticles. Sci Rep. 7:7933. DOI: 10.1038/s41598-017-08328-9. PMID: 28801679. PMCID: PMC5554140.
Article9. Peaper DR and Landry ML. 2014; Rapid diagnosis of influenza: state of the art. Clin Lab Med. 34:365–85. DOI: 10.1016/j.cll.2014.02.009. PMID: 24856533. PMCID: PMC7172071.10. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992; Evo-lution and ecology of influenza A viruses. Microbiol Mol Biol Rev. 56:152–79. DOI: 10.1128/MMBR.56.1.152-179.1992. PMID: 1579108. PMCID: PMC372859.
Article11. Kim JS, Lee SK, Ko DH, Hyun J, Kim HS. 2019; Performance evaluation of the automated fluorescent immunoassay system rotavirus assay in clinical samples. Ann Lab Med. 39:50–7. DOI: 10.3343/alm.2019.39.1.50. PMID: 30215230. PMCID: PMC6143470.
Article12. Ryu JH, Kwon M, Moon JD, Hwang MW, Lee JM, Park KH, et al. 2018; Development of a rapid automated fluorescent lateral flow immunoassay to detect hepatitis B surface antigen (HBsAg), antibody to HBsAg, and antibody to hepatitis C. Ann Lab Med. 38:578–84. DOI: 10.3343/alm.2018.38.6.578. PMID: 30027702. PMCID: PMC6056386.
Article13. Yoo Y, Sohn JW, Park DW, Kim JY, Shin HK, Lee Y, et al. 2007; Clinical evaluation of the SD Bioline influenza virus antigen test for rapid detection of influenza viruses A and B in children and adults during the influenza season. Clin Vaccine Immunol. 14:1050–2. DOI: 10.1128/CVI.00465-06. PMID: 17567765. PMCID: PMC2044486.
Article14. Ryu SW, Suh IB, Ryu SM, Shin KS, Kim HS, Kim J, et al. 2018; Comparison of three rapid influenza diagnostic tests with digital readout systems and one conventional rapid influenza diagnostic test. J Clin Lab Anal. 32:e22234. DOI: 10.1002/jcla.22234. PMID: 28407318. PMCID: PMC6817280.
Article15. Self WH, McNaughton CD, Grijalva CG, Zhu Y, Chappell JD, Williams JV, et al. 2012; Diagnostic performance of the BinaxNow Influenza A&B rapid antigen test in ED patients. Am J Emerg Med. 30:1955–61. DOI: 10.1016/j.ajem.2012.04.018. PMID: 22795995.16. Mese S, Akan H, Badur S, Uyanik A. Istanbul Rapid Test Study Group. 2016; Analytical performance of the BD Veritor™ System for rapid detection of influenza virus A and B in a primary healthcare setting. BMC Infect Dis. 16:481. DOI: 10.1186/s12879-016-1811-9. PMID: 27612949. PMCID: PMC5016879.
Article17. Hassan F, Nguyen A, Formanek A, Bell JJ, Selvarangan R. 2014; Comparison of the BD Veritor™ System for Flu A+B with the Alere BinaxNOW® In-fluenza A&B card for detection of influenza A and B viruses in respiratory specimens from pediatric patients. J Clin Microbiol. 52:906–10. DOI: 10.1128/JCM.02484-13. PMID: 24391198. PMCID: PMC3957768.18. Ryu SW, Lee JH, Kim J, Jang MA, Nam JH, Byoun MS, et al. 2016; Comparison of two new generation influenza rapid diagnostic tests with instrument-based digital readout systems for influenza virus detection. Br J Biomed Sci. 73:115–20. DOI: 10.1080/09674845.2016.1189026. PMID: 27327199.
Article19. Dunn J, Obuekwe J, Baun T, Rogers J, Patel T, Snow L. 2014; Prompt detec-tion of influenza A and B viruses using the BD Veritor™ System Flu A+B, Quidel® Sofia® Influenza A+B FIA, and Alere BinaxNOW® Influ-enza A&B compared to real-time reverse transcription-polymerase chain reaction (RT-PCR). Diagn Microbiol Infect Dis. 79:10–3. DOI: 10.1016/j.diagmicrobio.2014.01.018. PMID: 24582581.20. Selove W and Rao LV. 2016; Performance of rapid SOFIA Influenza A+B test compared to Luminex x-TAG respiratory viral panel assay in the diagnosis of influenza A, B, and subtype H3. J Investig Med. 64:905–7. DOI: 10.1136/jim-2016-000055. PMID: 26911275. PMCID: PMC4819670.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Evaluation of the STANDARD F Influenza A/B FIA Test with the Sofia Influenza A+B FIA and SD BIOLINE Influenza Ag A/B/A(H1N1) tests for Influenza A Virus Detection
- Evaluation of the Performance of a New Chromatographic Assay BD Veritor System for Rapid Detection of Influenza A & B
- The Efficacy of Rapid Antigen Tests for Detection of Seasonal Influenza Virus
- Methods for Evaluating the Accuracy of Diagnostic Tests
- Effect of rapid influenza diagnostic tests on patient management in an emergency department