Lab Med Online.
2020 Apr;10(2):116-124. 10.3343/lmo.2020.10.2.116.
Validation of Temperature Preservation in Specimen Transportation Systems
- Affiliations
-
- 1Department of Laboratory Medicine, Green Cross Laboratories, Yongin, Korea
- 2Department of Laboratory Medicine, Samkwang Medical Laboratories, Seoul, Korea
- 3Department of Laboratory Medicine, Seoul Clinical Laboratories, Yongin, Korea
- 4Department of Laboratory Medicine, Seegene Medical Foundation, Seoul, Korea
- 5Department of Laboratory Medicine, EONE Laboratories, Incheon, Korea
- 6Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea
- KMID: 2512241
- DOI: http://doi.org/10.3343/lmo.2020.10.2.116
Abstract
- Background
Clinical specimens are valuable materials that require a traceable management system. Maintenance of temperature and loss prevention during transport are important for the reliability of the clinical test results. Current transportation systems can suffer from temperature changes and agitation. Quality improvement in this pre-analytic phase is required. This study acquired preliminary data from a newly developed specimen transportation system adopting a real-time temperature monitoring during transportation using temperature sensor and global positioning system to establish appropriate guidelines.
Methods
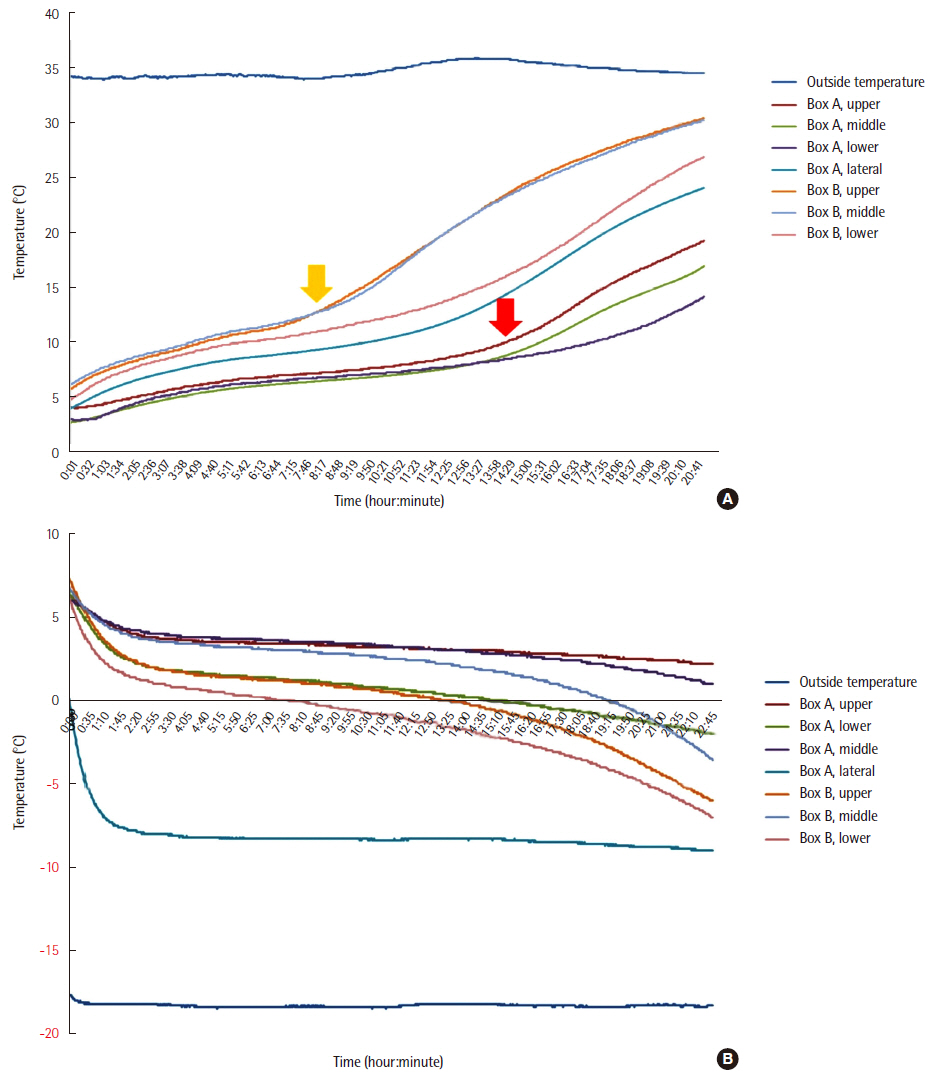
Temperature preservation performance was compared between two transportation boxes (newly developed one [A] and conventional one [B]) at exterior temperatures of 35℃ and ?18℃, reflecting the extreme temperature range in Korea. Influences of the temperatures on analytical results of whole blood, serum, plasma, and urine specimens were investigated, as were the effects of vibration.
Results
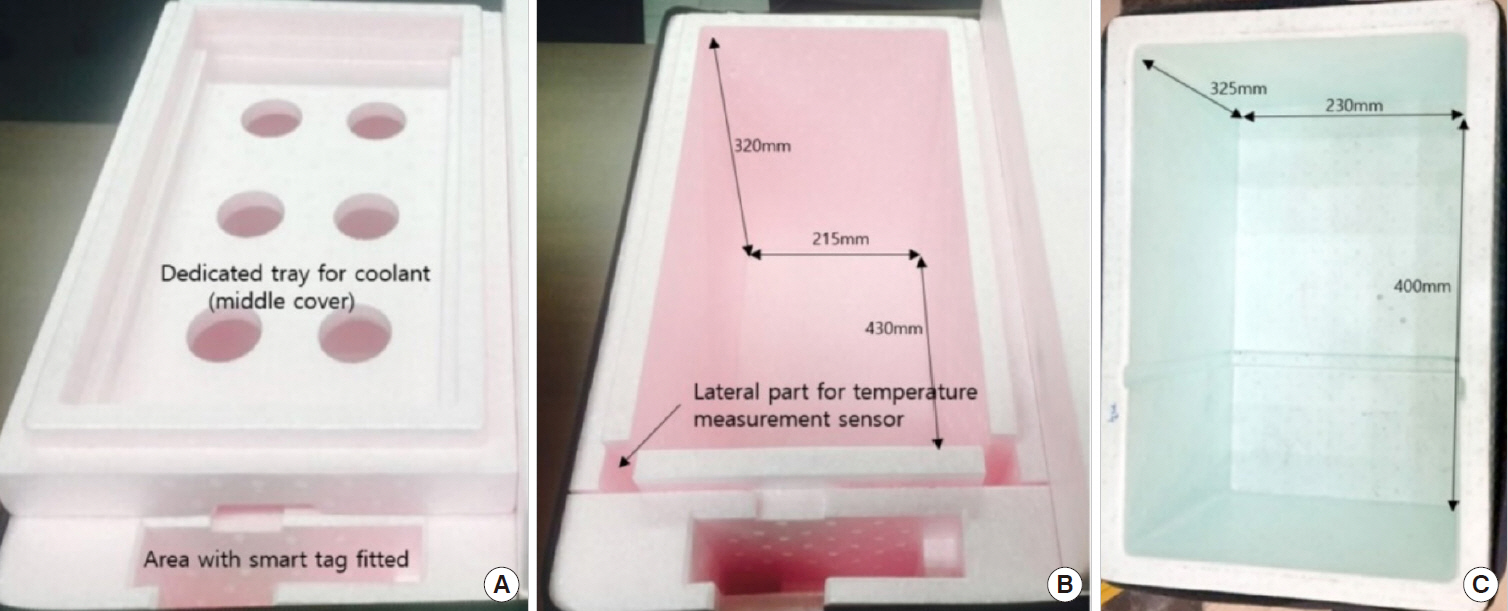
The interior temperature of box A measured at multiple sites was maintained within 1.0?9.0℃ at both exterior temperatures. The interior temperature of box B was outside of this range. The analyzed parameters varied comparably with the variations occurring at the recommended and published storage temperature. Vibration affected nonspecific enolase and lactate dehydrogenase.
Conclusions
Temperature preservation and real-time monitoring during specimen transportation are important. The present data highlight the importance of transportation conditions and indicate that laboratories should know the characteristics of temperature changes in their transportation system.
Keyword
Figure
Reference
-
1. Lim HS, Lee YK, Min WK. 2011; Effects of long distance transportation of specimens on test results. Lab Med Online. 1:72–80. DOI: 10.3343/lmo.2011.1.2.2.
Article2. Desirable biological variation database specification. www.westgard.com/biodatabase1.htm. updated on 2014.3. Kim SK, Jeong TD, Lee W, Chun S, Min WK. 2015; Performance evaluation of the Elecsys neuron-specific enolase. Lab Med Online. 5:63–8. DOI: 10.3343/lmo.2015.5.2.63.4. Royal College of Pathologists of Australasia (RCPA) analytical quality requirements. https://www.westgard.com/rcpa-biochemistry.htm. Updated on Aug 2010.5. Goyal T, Schmotzer CL. 2015; Validation of hemolysis index threshold optimizes detection of clinically significant hemolysis. Am J Clin Pathol. 143:579–83. DOI: 10.1309/AJCPDUDE1HRA0YMR. PMID: 25780011.6. Westgard JO. 2008. Basic method validation: Training in analytical quality management for healthcare laboratories. 3rd ed. WI: Westgard QC;Madison: p. 8–9.7. Plebani M. 2006; Errors in clinical laboratories or errors in laboratory medicine. Clin Chem Lab Med. 44:750–9. DOI: 10.1515/CCLM.2006.123. PMID: 16729864.
Article8. Kim HK, Kim BJ, Jeong KY, Cheon SS. 2013; Experimental study for the im-pact characteristics of expanded EPP/EPS foams. J Korean Soc Comp Mat. 26:343–8. DOI: 10.7234/composres.2013.26.6.343.
Article9. Korean Agency for Technology and Standards, KS M3808. 2011. Cellular polystyrene (PS) for thermal insulation. https://www.standard. go.kr/KSCI/standardIntro/getStandardSearchView.do?.menuId=919&topMenuId=502&upperMenuId=503&ksNo=KSM3808&tmprKsNo=KS M3808&reformNo=24.10. Sutterlin WR. Phase change materials, a brief comparison of ice packs, salts, parafns, and vegetable-derived phase change materials. http://www.pharmoutsourcing.com/Featured-Articles/37854-Phase-Change-Materials-A-Brief-Comparison-of-Ice-Packs-Salts-Parafns-and-Vegetable-derived-Phase-Change-Materials. Updated on Jul 2011.11. Sharma A, Tyagi VV, Chen CR, Buddhi D. 2009; Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev. 13:318–45. DOI: 10.1016/j.rser.2007.10.005.
Article12. Wood BL, Andrews J, Miller S, Sabath DE. 1999; Refrigerated storage impro-ves the stability of the complete blood cell count and automated differential. Am J Clin Pathol. 112:687–95. DOI: 10.1093/ajcp/112.5.687. PMID: 10549256.
Article13. Clinical and Laboratory Standards Institute. 2010; Procedures for the han-dling and processing of blood specimens for common laboratory tests; Approved guideline-Fourth edition. CLSI document H18-A4. Clinical and Laboratory Standards Institute;Wayne, PA:14. Mayo Clinic Laboratories. International shipping guide: Shipping specimens from international locations. https://www.mayomedicallaboratories.com/specimen/transport/index.php. Last accessed by 2016.15. World Health Organization. Guidance on regulations for the transport of infectious substances 2017-2018. http://apps.who.int/iris/bitstream/10665/254788/1/WHO-WHE-CPI-2017.8-eng.pdf?ua=1. Updated on 2017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Long Distance Transportation of Specimens on Test Results
- Air Travel and Transportation of Patients (I) A guide for physicians
- Air Travel and Transportation of Patients (IV): A guide for physicians
- Air Travel and Transportation of Patients (III): A guide for physicians
- Inspection and Evaluation of Blood Cold Chain