J Pathol Transl Med.
2020 Nov;54(6):480-488. 10.4132/jptm.2020.07.18.
Evaluation of human papillomavirus (HPV) prediction using the International Endocervical Adenocarcinoma Criteria and Classification system, compared to p16 immunohistochemistry and HPV RNA in-situ hybridization
- Affiliations
-
- 1Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada
- 2Genetic Pathology Evaluation Center (GPEC), Vancouver, BC, Canada
- 3Department of Pathology, University of Medicine, Pharmacy, Sciences and Technology of Targu Mures, Targu Mures, Romania
- 4Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
- 5Molecular Oncology, British Columbia Cancer Research Centre, Vancouver, BC, Canada
- 6Department of Anatomical Pathology, Vancouver General Hospital, Vancouver, BC, Canada
- KMID: 2509501
- DOI: http://doi.org/10.4132/jptm.2020.07.18
Abstract
- Background
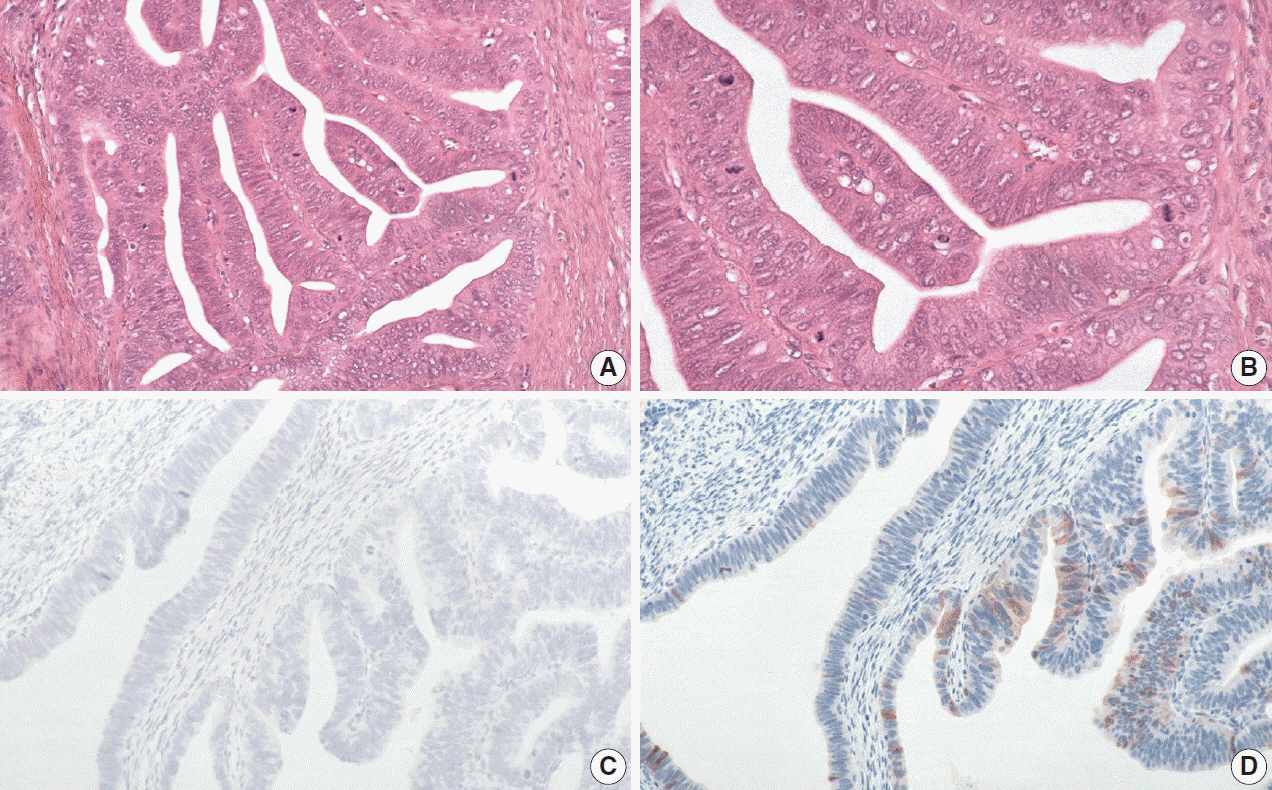
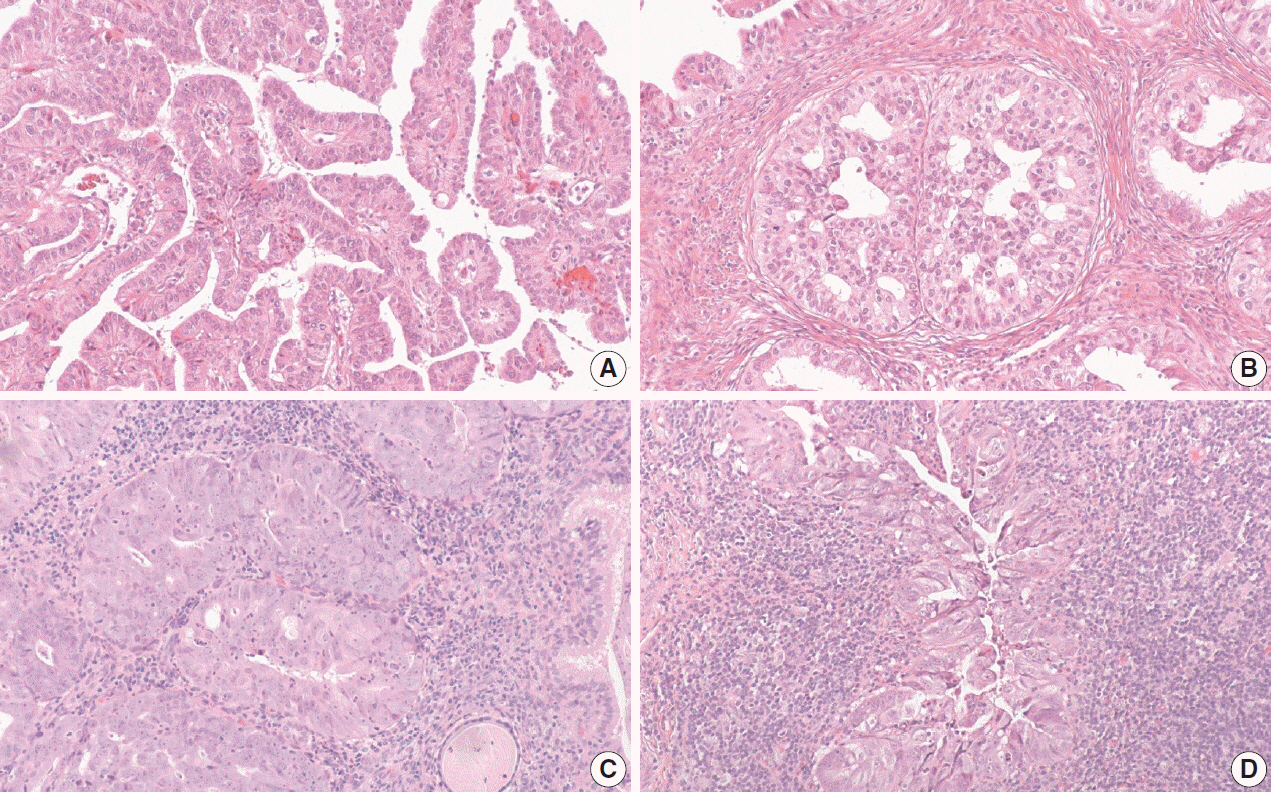
The International Endocervical Adenocarcinoma Criteria and Classification (IECC) separated endocervical adenocarcinomas into human papillomavirus (HPV) associated (HPVA) and non–HPV-associated (NHPVA) categories by morphology alone. Our primary objective was to assess the accuracy of HPV prediction by the IECC system compared to p16 immunohistochemistry and HPV RNA in-situ hybridization (RISH). Our secondary goal was to directly compare p16 and HPV RISH concordance.
Methods
Cases were classified by IECC and stained for p16 and HPV RISH on tissue microarray, with discordant p16/HPV RISH cases re-stained on whole tissue sections. Remaining discordant cases (p16/HPV, IECC/p16, IECC/HPV discordances) were re-reviewed by the original pathologists (n = 3) and external expert pathologists (n = 2) blinded to the p16 and HPV RISH results. Final IECC diagnosis was assigned upon independent agreement between all reviewers.
Results
One hundred and eleven endocervical adenocarcinomas were classified originally into 94 HPVA and 17 NHPVA cases. p16 and HPV RISH was concordant in 108/111 cases (97%) independent of the IECC. HPV RISH and p16 was concordant with IECC in 103/111 (93%) and 106/111 (95%), respectively. After expert review, concordance improved to 107/111 (96%) for HPV RISH. After review of the eight discordant cases, one remained as HPVA, four were reclassified to NHPVA from HPVA, two were unclassifiable, and one possibly represented a mixed usual and gastric-type adenocarcinoma.
Conclusions
p16 and HPV RISH have excellent concordance in endocervical adenocarcinomas, and IECC can predict HPV status in most cases. Focal apical mitoses and apoptotic debris on original review led to the misclassification of several NHPVA as HPVA.
Keyword
Figure
Reference
-
References
1. Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health (Larchmt). 2012; 21:1031–7.
Article2. Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States: a 24-year population-based study. Gynecol Oncol. 2000; 78:97–105.3. Stolnicu S, Hoang L, Soslow RA. Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. 2019; 475:537–49.
Article4. McAlpine JN, Leung SC, Cheng A, et al. Human papillomavirus (HPV)-independent vulvar squamous cell carcinoma has a worse prognosis than HPV-associated disease: a retrospective cohort study. Histopathology. 2017; 71:238–46.
Article5. Nooij LS, Ter Haar NT, Ruano D, et al. Genomic characterization of vulvar (pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin Cancer Res. 2017; 23:6781–9.
Article6. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010; 363:24–35.
Article7. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008; 100:261–9.
Article8. Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010; 28:4142–8.9. Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011; 22:1071–7.
Article10. Hodgson A, Park KJ, Djordjevic B, et al. International endocervical adenocarcinoma criteria and classification: validation and interobserver reproducibility. Am J Surg Pathol. 2019; 43:75–83.11. Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. 2018; 42:214–26.12. Prigge ES, Arbyn M, von Knebel Doeberitz M, Reuschenbach M. Diagnostic accuracy of p16(INK4a) immunohistochemistry in oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis. Int J Cancer. 2017; 140:1186–98.13. Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012; 36:945–54.
Article14. Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012; 14:22–9.15. Schache AG, Liloglou T, Risk JM, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013; 108:1332–9.
Article16. Keung ES, Souers RJ, Bridge JA, et al. Comparative performance of high-risk human papillomavirus RNA and DNA in situ hybridization on college of American pathologists proficiency tests. Arch Pathol Lab Med. 2020; 144:344–9.
Article17. Satgunaseelan L, Chia N, Suh H, et al. p16 expression in cutaneous squamous cell carcinoma of the head and neck is not associated with integration of high risk HPV DNA or prognosis. Pathology. 2017; 49:494–8.
Article18. Augustin J, Outh-Gauer S, Mandavit M, et al. Evaluation of the efficacy of the 4 tests (p16 immunochemistry, polymerase chain reaction, DNA, and RNA in situ hybridization) to evaluate a human papillomavirus infection in head and neck cancers: a cohort of 348 French squamous cell carcinomas. Hum Pathol. 2018; 78:63–71.
Article19. Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012; 36:1874–82.
Article20. Evans MF, Peng Z, Clark KM, et al. HPV E6/E7 RNA in situ hybridization signal patterns as biomarkers of three-tier cervical intraepithelial neoplasia grade. PLoS One. 2014; 9:e91142.
Article21. Roldan Urgoiti GB, Gustafson K, Klimowicz AC, Petrillo SK, Magliocco AM, Doll CM. The prognostic value of HPV status and p16 expression in patients with carcinoma of the anal canal. PLoS One. 2014; 9:e108790.
Article22. Zappacosta R, Colasante A, Viola P, et al. Chromogenic in situ hybridization and p16/Ki67 dual staining on formalin-fixed paraffinembedded cervical specimens: correlation with HPV-DNA test, E6/E7 mRNA test, and potential clinical applications. Biomed Res Int. 2013; 2013:453606.
Article23. Aumayr K, Susani M, Horvat R, et al. P16INK4A immunohistochemistry for detection of human papilloma virus-associated penile squamous cell carcinoma is superior to in-situ hybridization. Int J Immunopathol Pharmacol. 2013; 26:611–20.24. Winters R, Trotman W, Adamson CS, et al. Screening for human papillomavirus in basaloid squamous carcinoma: utility of p16(INK4a), CISH, and PCR. Int J Surg Pathol. 2011; 19:309–14.
Article25. Evans MF, Matthews A, Kandil D, Adamson CS, Trotman WE, Cooper K. Discrimination of ‘driver’ and ‘passenger’ HPV in tonsillar carcinomas by the polymerase chain reaction, chromogenic in situ hybridization, and p16(INK4a) immunohistochemistry. Head Neck Pathol. 2011; 5:344–8.
Article26. Sheng Z, Minato H, Sasagawa T, et al. Detection of high-risk human papillomavirus subtypes in cervical glandular neoplasia by in situ hybridization. Int J Clin Exp Pathol. 2013; 6:2168–77.27. Chen T, Li J, Wang S, Ning Y, Zhou X, Wang Y. High-risk HPV E6/E7 mRNA in situ hybridization in endocervical glandular neoplasia: performance compared with p16(INK4a) and Ki67 immunochemistry. Am J Transl Res. 2019; 11:6498–506.28. Peng WX, Kure S, Ishino K, et al. P16-positive continuous minimal deviation adenocarcinoma and gastric type adenocarcinoma in a patient with Peutz-Jeghers syndrome. Int J Clin Exp Pathol. 2015; 8:5877–82.29. Houghton O, Jamison J, Wilson R, Carson J, McCluggage WG. p16 Immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology. 2010; 57:342–50.
Article30. Lu S, Shen D, Zhao Y, Kang N, Wang X. Primary endocervical gastric-type adenocarcinoma: a clinicopathologic and immunohistochemical analysis of 23 cases. Diagn Pathol. 2019; 14:72.
Article31. Stolnicu S, Barsan I, Hoang L, et al. Diagnostic algorithmic proposal based on comprehensive immunohistochemical evaluation of 297 invasive endocervical adenocarcinomas. Am J Surg Pathol. 2018; 42:989–1000.
Article32. Singh N, Gilks CB, Wong RW, McCluggage WG, Herrington CS. Interpretation of p16 immunohistochemistry in lower anogenital tract neoplasia [Internet]. Derby: British Association of Gynaecological Pathologists;2018. [cited 2019 Dec 11]. Available from: https://www.bgcs.org.uk/wp-content/uploads/2019/05/BAGP-UKNEQAScIQC-project-p16-interpretation-guide-2018.pdf .33. Wada T, Ohishi Y, Kaku T, et al. Endocervical adenocarcinoma with morphologic features of both usual and gastric types: clinicopathologic and immunohistochemical analyses and high-risk HPV detection by in situ hybridization. Am J Surg Pathol. 2017; 41:696–705.34. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003; 16:1–17.
Article35. Poetsch M, Hemmerich M, Kakies C, et al. Alterations in the tumor suppressor gene p16(INK4A) are associated with aggressive behavior of penile carcinomas. Virchows Arch. 2011; 458:221–9.36. Nuovo GJ, Plaia TW, Belinsky SA, Baylin SB, Herman JG. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc Natl Acad Sci U S A. 1999; 96:12754–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Papillomavirus 16/18 Expression of Endocervical Glandular Lesions: Relationship with p53 and MIB-1 Expressions
- Gastric type mucinous endocervical adenocarcinoma of the uterine cervix: very rare and interesting case
- Detection of Human Papilloma Virus Type 16 and 18 in Adenocarcinoma in situ of the Uterine Cervix
- Human Papillomavirus Genotyping and p16INK4a Expression in Cervical Lesions: A Combined Test to Avoid Cervical Cancer Progression
- Expression of Human Papillomavirus-Related Proteins and Its Clinical Implication in Tonsillar Squamous Cell Carcinoma