Int J Stem Cells.
2020 Nov;13(3):305-311. 10.15283/ijsc20076.
Emerging Treatment Options of Regenerative Medicine in Severe Corona Virus/COVID 19 Infections
- Affiliations
-
- 1Department of Orthopedics, My Doc Specialist Medical Centre DMCC, Dubai, UAE
- 2Department of Orthopedics, Saudi German Hospital, Dubai, UAE
- 3HOD of Department of Obs-Gynae, Saudi German Hospital, Dubai, UAE
- KMID: 2508904
- DOI: http://doi.org/10.15283/ijsc20076
Abstract
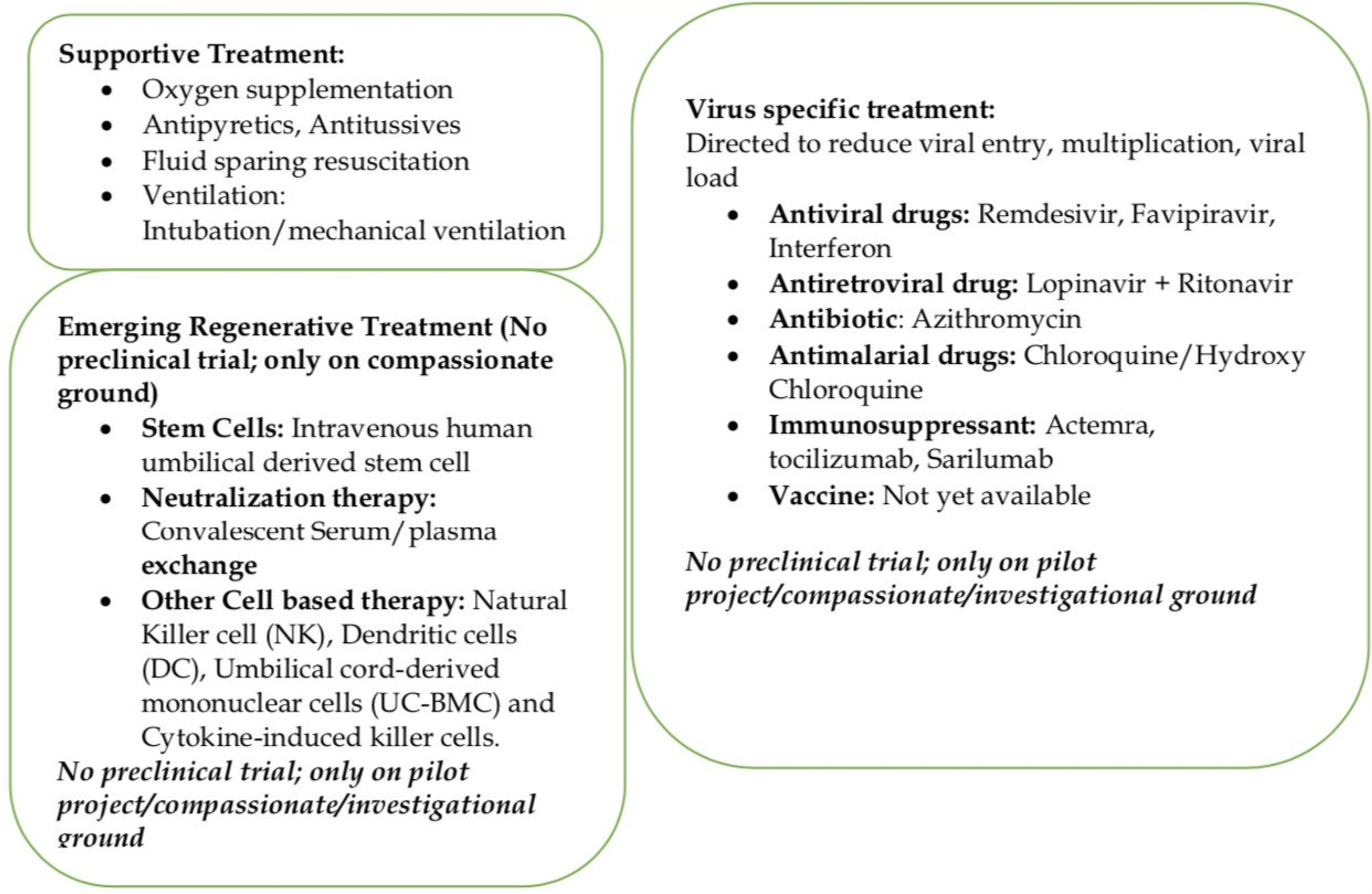
- COVID-19 pandemic has brought the whole world stand still, locked down in their homes, infecting more than 8 million people, and many thousands (449,182) -have lost their lives across the globe. Due to lack of any definitive medicine or vaccine, treatment options are supportive of oxygenation, antiviral, antiretroviral drugs, antibiotics, fluid/electrolyte, mechanical ventilation with ICU (Intensive Care Unit) support, and hloroquine/hydroxychloroquine have been tried to fight this infection. However, mortality due to severe pneumonia, ARDS (Acute Respiratory Distress Syndrome), and multiorgan failure arising from the overactive immune response (storm) mediated by cytokines remains a treatment challenge in elderly and patients with severe medical comorbidities. Recently, anti-inflammatory, angiogenic, immune-modular, and healing properties of intravenous injections of culture derived stem cells have been proposed and shown to benefits in a small number of patients with severe COVID-19 infections. Based on previous experience with other viral infections, convalescent plasma, and serum transfusion are being used as a source of neutralizing antibody/factors to minimize the effects of inflammatory cytokines in this infection. Immunotherapy with purified monoclonal antibodies and conditioned serum with a mixture of unique cytokines are also being developed. Regenerative Medicine has emerged as a crucial adjuvant tool in promoting healing and early recovery in severe COVID-19 infections and other supportive treatments.
Figure
Reference
-
References
1. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. 2020; The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 5:536–544. DOI: 10.1038/s41564-020-0695-z. PMID: 32123347. PMCID: PMC7095448.2. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. 2020; Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 382:1199–1207. DOI: 10.1056/NEJMoa2001316. PMID: 31995857. PMCID: PMC7121484.
Article3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020; Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395:497–506. DOI: 10.1016/S0140-6736(20)30183-5. PMID: 31986264. PMCID: PMC7159299.
Article4. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. 2020; A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 395:514–523. DOI: 10.1016/S0140-6736(20)30154-9. PMID: 31986261. PMCID: PMC7159286.
Article5. Burke RM, Midgley CM, Dratch A, Fenstersheib M, Haupt T, Holshue M, Ghinai I, Jarashow MC, Lo J, McPherson TD, Rudman S, Scott S, Hall AJ, Fry AM, Rolfes MA. 2020; Active monitoring of persons exposed to patients with confirmed COVID-19-United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 69:245–246. DOI: 10.15585/mmwr.mm6909e1. PMID: 32134909. PMCID: PMC7367094.
Article6. Chen Y, Guo Y, Pan Y, Zhao ZJ. 2020; Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 525:135–140. DOI: 10.1016/j.bbrc.2020.02.071. PMID: 32081428. PMCID: PMC7092824.
Article7. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. 2020; Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 367:1444–1448. DOI: 10.1126/science.abb2762. PMID: 32132184. PMCID: PMC7164635.
Article8. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. 2020; Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 14:185–192. DOI: 10.1007/s11684-020-0754-0. PMID: 32170560. PMCID: PMC7088738.
Article9. Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. 2020; Mohareb AM. Is there an association between COVID-19 mortality and the renin-angiotensin system-a call for epidemiologic investigations. Clin Infect Dis. doi:10.1093/cid/ciaa329. [Epub ahead of print]. DOI: 10.1093/cid/ciaa329. PMID: 32215613. PMCID: PMC7184340.10. 2020. Jun. 25. People who are at increased risk for severe illness [Internet]. Centers for Disease Control and Prevention;Atlanta: Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-increased-risk.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fpeople-at-higher-risk.html. updated 2020 Jun 25; cited 2020 Jun 25.11. World Health Organization. 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. World Health Organization;Geneva: DOI: 10.15557/PiMR.2020.0003.12. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. 2020; Hydroxychlo-roquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. doi:10.1016/j.ijantimicag.2020.105 949. [Epub ahead of print]. DOI: 10.1016/j.ijantimicag.2020.105949. PMID: 32205204. PMCID: PMC7102549.
Article13. Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC. 2020; Epide-miologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 323:1488–1494. DOI: 10.1001/jama.2020.3204. PMID: 32125362. PMCID: PMC7054855.
Article14. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. 2020; A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 382:1787–1799. DOI: 10.1056/NEJMoa2001282. PMID: 32187464. PMCID: PMC7121492.
Article15. Gupta PK, Das AK, Chullikana A, Majumdar AS. 2012; Mesen-chymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 3:25. DOI: 10.1186/scrt116. PMID: 22776206. PMCID: PMC3580463.
Article16. Murphy JM, Fink DJ, Hunziker EB, Barry FP. 2003; Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48:3464–3474. DOI: 10.1002/art.11365. PMID: 14673997.
Article17. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. 2004; Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 109:1292–1298. DOI: 10.1161/01.CIR.0000121425.42966.F1. PMID: 14993122.
Article18. Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ. 2009; Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 27:670–681. DOI: 10.1002/stem.20080742. PMID: 19267325. PMCID: PMC4742302.
Article19. Caplan AI. 2009; Why are MSCs therapeutic? New data: new insight. J Pathol. 217:318–324. DOI: 10.1002/path.2469. PMID: 19023885.
Article20. Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. 2016; Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy-a review. BMC Musculoskelet Disord. 17:230. DOI: 10.1186/s12891-016-1085-9. PMID: 27229856. PMCID: PMC4880954.21. Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. 2019; Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 109:2318–2326. DOI: 10.1016/j.biopha.2018.11.099. PMID: 30551490.
Article22. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. 2009; Intra-venous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5:54–63. DOI: 10.1016/j.stem.2009.05.003. PMID: 19570514. PMCID: PMC4154377.
Article23. Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. 2020; Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 55:2000858. DOI: 10.1183/13993003.00858-2020. PMID: 32265310. PMCID: PMC7144273.
Article24. Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee JW. 2018; Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 7:615–624. DOI: 10.1002/sctm.17-0278. PMID: 29737632. PMCID: PMC6090509.
Article25. Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. 2010; Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 28:2229–2238. DOI: 10.1002/stem.544. PMID: 20945332. PMCID: PMC3293245.
Article26. Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F. 2008; Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 26:279–289. DOI: 10.1634/stemcells.2007-0454. PMID: 17962701.
Article27. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. 2010; A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immuno-suppressive MSC2 phenotype. PLoS One. 5:e10088. DOI: 10.1371/journal.pone.0010088. PMID: 20436665. PMCID: PMC2859930.
Article28. Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. 2013; Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 187:751–760. DOI: 10.1164/rccm.201206-0990OC. PMID: 23292883. PMCID: PMC3678109.
Article29. Behnke J, Kremer S, Shahzad T, Chao CM, Böttcher-Friebertshäuser E, Morty RE, Bellusci S, Ehrhardt H. 2020; MSC based therapies-new perspectives for the injured lung. J Clin Med. 9:682. DOI: 10.3390/jcm9030682. PMID: 32138309. PMCID: PMC7141210.
Article30. Cruz FF, Rocco PRM. 2020; The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med. 14:31–39. DOI: 10.1080/17476348.2020.1679628. PMID: 31608724.
Article31. Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. 2016; Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 14:1681–1692. DOI: 10.3892/mmr.2016.5416. PMID: 27357811.
Article32. Iyer SS, Co C, Rojas M. 2009; Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 51:5–16. PMID: 19352305.33. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. 2020; Trans-plantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 11:216–228. DOI: 10.14336/AD.2020.0228. PMID: 32257537. PMCID: PMC7069465.
Article34. Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Nie P, Gao Y, Qian C, Hu M. 2020; Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. ChinaXiv. doi:10.12074/202002.00084. DOI: 10.1097/MD.0000000000021429. PMID: 32756149. PMCID: PMC7402800.
Article35. Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. 2017; Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopa-thy]). Circ Res. 121:1192–1204. DOI: 10.1161/CIRCRESAHA.117.310712. PMID: 28974553. PMCID: PMC6372053.
Article36. González PL, Carvajal C, Cuenca J, Alcayaga-Miranda F, Figueroa FE, Bartolucci J, Salazar-Aravena L, Khoury M. 2015; Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Transl Med. 4:1109–1121. DOI: 10.5966/sctm.2015-0022. PMID: 26273064. PMCID: PMC4572900.
Article37. Slater H. 2020. Apr. 3. FDA accepts IND for NK cell therapy CYNK-001 to treat patients with COVID-19 [Internet]. Cancer Network;Cranbury: Available from: https://www.cancernetwork.com/view/fda-accepts-ind-nk-cell-therapy-cynk-001-treat-patients-covid-19. cited 2020 Apr 3.38. Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. 2005; Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 24:44–46. DOI: 10.1007/s10096-004-1271-9. PMID: 15616839. PMCID: PMC7088355.
Article39. Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, de la Calle F, Borobia AM, Sánchez-Seco P, Lago M, Figueira JC, Fernández-Puntero B, Viejo A, Negredo A, Nuñez C, Flores E, Carcas AJ, Jiménez-Yuste V, Lasala F, García-de-Lorenzo A, Arnalich F, Arribas JR. La Paz-Carlos III University Hospital Isolation Unit. 2015; Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 3:554–562. DOI: 10.1016/S2213-2600(15)00180-0. PMID: 26041403.
Article40. Benson AB, Moss M, Silliman CC. 2009; Transfusion-related acute lung injury (TRALI): a clinical review with emphasis on the critically ill. Br J Haematol. 147:431–443. DOI: 10.1111/j.1365-2141.2009.07840.x. PMID: 19663827. PMCID: PMC4532338.
Article41. Patel P, Nandwani V, Vanchiere J, Conrad SA, Scott LK. 2011; Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A--an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 12:e87–e89. DOI: 10.1097/PCC.0b013e3181e2a569. PMID: 20453703. PMCID: PMC6328374.
Article42. Chang JC. 2019; Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 17:10. DOI: 10.1186/s12959-019-0198-4. PMID: 31160889. PMCID: PMC6542012.
Article43. Halstead SB. 2014; Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiol Spectr. doi:10.1128/microbiolspec.AID-0022-2014. DOI: 10.1128/microbiolspec.AID-0022-2014. PMID: 26104444.
Article44. Nguyen TC, Carcillo JA. 2006; Bench-to-bedside review: thrombocytopenia-associated multiple organ failure--a newly appreciated syndrome in the critically ill. Crit Care. 10:235. DOI: 10.1186/cc5064. PMID: 17096864. PMCID: PMC1794442.
Article45. Knaup H, Stahl K, Schmidt BMW, Idowu TO, Busch M, Wiesner O, Welte T, Haller H, Kielstein JT, Hoeper MM, David S. 2018; Early therapeutic plasma exchange in septic shock: a prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care. 22:285. DOI: 10.1186/s13054-018-2220-9. PMID: 30373638. PMCID: PMC6206942.
Article46. Busund R, Koukline V, Utrobin U, Nedashkovsky E. 2002; Plas-mapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med. 28:1434–1439. DOI: 10.1007/s00134-002-1410-7. PMID: 12373468.
Article