Nutr Res Pract.
2020 Dec;14(6):593-605. 10.4162/nrp.2020.14.6.593.
Paeoniflorin ameliorates Aβ-stimulated neuroinflammation via regulation of NF-κB signaling pathway and Aβ degradation in C6 glial cells
- Affiliations
-
- 1Department of Food Science and Nutrition & Kimchi Research Institute, Pusan National University, Busan 46241, Korea
- 2Department of Food Science, Gyeongnam National University of Science and Technology, Jinju 52725, Korea
- KMID: 2508793
- DOI: http://doi.org/10.4162/nrp.2020.14.6.593
Abstract
- BACKGROUND/OBJECTIVES
Alzheimer's disease is common age-related neurodegenerative condition characterized by amyloid beta (Aβ) accumulation that leads cognitive impairment. In the present study, we investigated the protective effect of paeoniflorin (PF) against Aβ-induced neuroinflammation and the underlying mechanism in C6 glial cells.
MATERIALS/METHODS
C6 glial cells were treated with PF and Aβ25–35, and cell viability, nitric oxide (NO) production, and pro-inflammatory cytokine release were measured. Furthermore, the mechanism underlying the effect of PF on inflammatory responses and Aβ degradation was determined by Western blot.
RESULTS
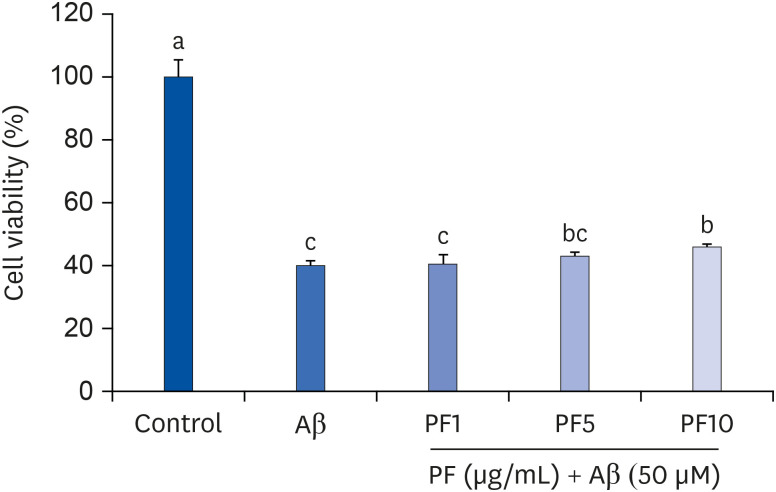
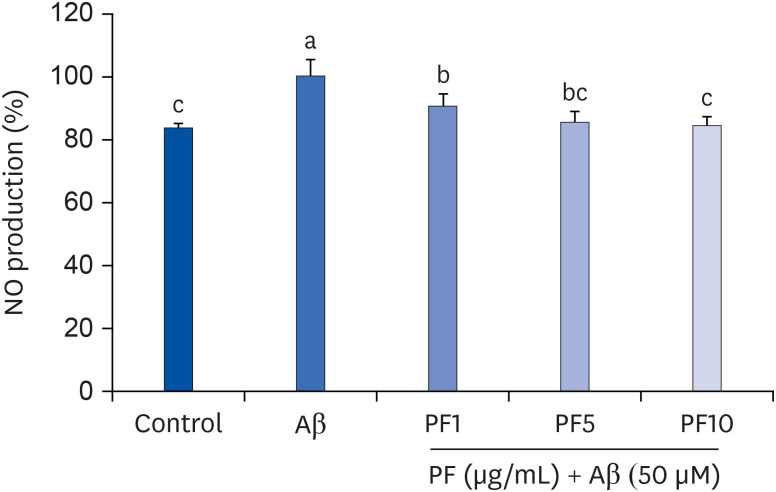
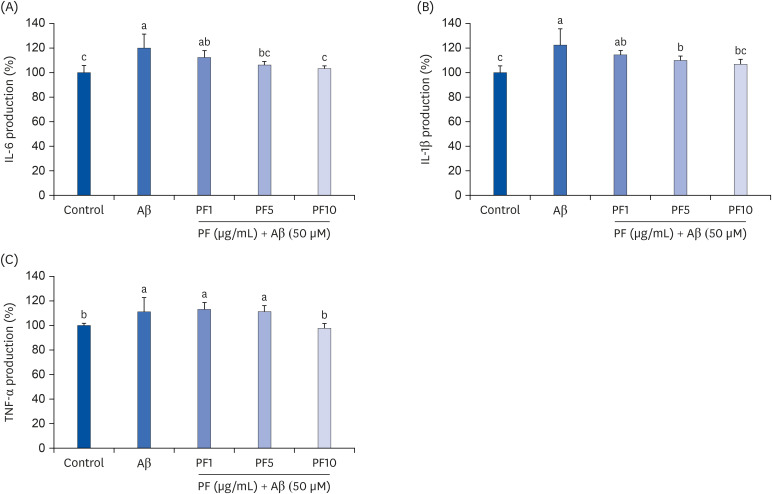
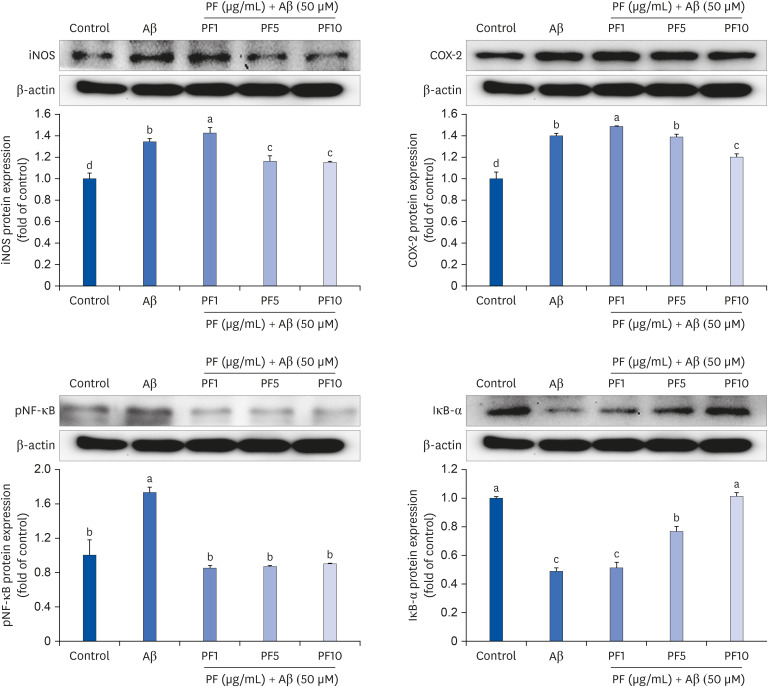
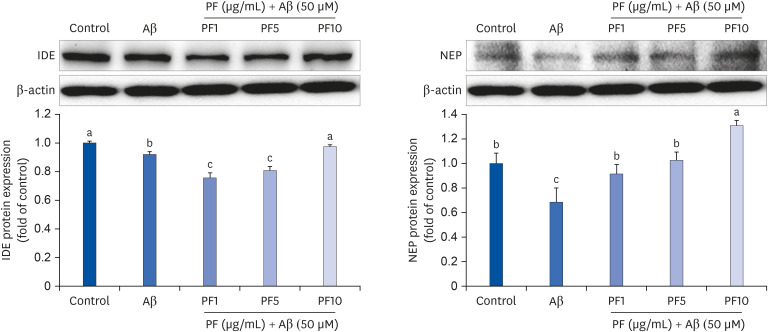
Aβ25–35 significantly reduced cell viability, but this reduction was prevented by the pretreatment with PF. In addition, PF significantly inhibited Aβ25–35 -induced NO production in C6 glial cells. The secretion of interleukin (IL)-6, IL-1β, and tumor necrosis factor-alpha was also significantly reduced by PF. Further mechanistic studies indicated that PF suppressed the production of these pro-inflammatory cytokines by regulating the nuclear factor-kappa B (NF-κB) pathway. The protein levels of inducible NO synthase and cyclooxygenase-2 were downregulated and phosphorylation of NF-κB was blocked by PF. However, PF elevated the protein expression of inhibitor kappa B-alpha and those of Aβ degrading enzymes, insulin degrading enzyme and neprilysin.
CONCLUSIONS
These findings indicate that PF exerts protective effects against Aβ-mediated neuroinflammation by inhibiting NF-κB signaling, and these effects were associated with the enhanced activity of Aβ degradation enzymes.
Keyword
Figure
Reference
-
1. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010; 119:7–35. PMID: 20012068.
Article2. Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003; 304:1–7. PMID: 12490568.
Article3. Pereira C, Agostinho P, Moreira PI, Cardoso SM, Oliveira CR. Alzheimer's disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr Drug Targets CNS Neurol Disord. 2005; 4:383–403. PMID: 16101556.
Article4. Avila-Muñoz E, Arias C. When astrocytes become harmful: functional and inflammatory responses that contribute to Alzheimer's disease. Ageing Res Rev. 2014; 18:29–40. PMID: 25078115.
Article5. Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ . Nat Med. 2003; 9:453–457. PMID: 12612547.6. Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A, Gonzales E, Burchett JM, Schuler DR, Cirrito JR, Diwan A, Lee JM. Enhancing astrocytic lysosome biogenesis facilitates Aβ clearance and attenuates amyloid plaque pathogenesis. J Neurosci. 2014; 34:9607–9620. PMID: 25031402.
Article7. Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008; 28:8354–8360. PMID: 18701698.
Article8. He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora pall., a traditional Chinese herbal medicine. Front Pharmacol. 2011; 2:10. PMID: 21687505.
Article9. Qiu F, Zhong X, Mao Q, Huang Z. The antidepressant-like effects of paeoniflorin in mouse models. Exp Ther Med. 2013; 5:1113–1116. PMID: 23599734.
Article10. Ikeda N, Fukuda T, Jyo H, Shimada Y, Murakami N, Saka M, Yoshikawa M. Quality evaluation on Paeoniae Radix. I. Quantitative analysis of monoterpene glycosides constituents of Paeoniae Radix by means of high performance liquid chromatography. Comparative characterization of the external figures, processing method and the cultivated areas. Yakugaku Zasshi. 1996; 116:138–147. PMID: 8717280.
Article11. Yoo JS, Song MC, Ahn EM, Lee YH, Rho YD, Baek NI. Quantitative analysis of paeoniflorin from Paeonia lactiflora using 1H-NMR. Nat Prod Sci. 2006; 12:237–240.12. Zhang MH, Feng L, Zhu MM, Gu JF, Wu C, Jia XB. Antioxidative and anti-inflammatory activities of paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell damage. Planta Med. 2013; 79:1319–1323. PMID: 23881455.
Article13. Wang QS, Gao T, Cui YL, Gao LN, Jiang HL. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol. 2014; 52:1189–1195. PMID: 24646307.14. Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F. Protective effect of paeoniflorin on Aβ25–35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol. 2014; 34:227–234. PMID: 24263411.15. Zhang Y, Li H, Huang M, Huang M, Chu K, Xu W, Zhang S, Que J, Chen L. Paeoniflorin, a monoterpene glycoside, protects the brain from cerebral ischemic injury via inhibition of apoptosis. Am J Chin Med. 2015; 43:543–557. PMID: 25967667.
Article16. Chen A, Wang H, Zhang Y, Wang X, Yu L, Xu W, Xu W, Lin Y. Paeoniflorin exerts neuroprotective effects against glutamate‑induced PC12 cellular cytotoxicity by inhibiting apoptosis. Int J Mol Med. 2017; 40:825–833. PMID: 28731183.
Article17. Nam MN, Lee AY, Sin SM, Goo YM, Choi JM, Cho EJ. Protective effects of Paeonia lactiflora and its active compound, paeoniflorin, against neuronal oxidative stress in H2O2-treated SH-SY5Y cells. Int J Gerontol. 2019; S39–44.18. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63. PMID: 6606682.
Article19. Dirsch VM, Stuppner H, Vollmar AM. The Griess assay: suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med. 1998; 64:423–426. PMID: 9690344.
Article20. McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995; 21:195–218. PMID: 8866675.
Article21. Qiu WQ, Ye Z, Kholodenko D, Seubert P, Selkoe DJ. Degradation of amyloid β-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. J Biol Chem. 1997; 272:6641–6646. PMID: 9045694.
Article22. Peters C, Bascuñán D, Opazo C, Aguayo LG. Differential membrane toxicity of amyloid-β fragments by pore forming mechanisms. J Alzheimers Dis. 2016; 51:689–699. PMID: 26890761.
Article23. Hughes E, Burke RM, Doig AJ. Inhibition of toxicity in the beta-amyloid peptide fragment beta -(25–35) using N-methylated derivatives: a general strategy to prevent amyloid formation. J Biol Chem. 2000; 275:25109–25115. PMID: 10825171.24. Liu Q, Zhao B. Nicotine attenuates beta-amyloid peptide-induced neurotoxicity, free radical and calcium accumulation in hippocampal neuronal cultures. Br J Pharmacol. 2004; 141:746–754. PMID: 14757701.25. Deng LJ, Cheng C, Wu J, Wang CH, Zhou HB, Huang J. Oxabicycloheptene sulfonate protects against β-amyloid-induced toxicity by activation of PI3K/Akt and ERK signaling pathways via GPER1 in C6 cells. Neurochem Res. 2017; 42:2246–2256. PMID: 28374135.
Article26. Zhao X, Yuan L, Yu H, Xi Y, Ma W, Zhou X, Ding J, Xiao R. Genistein inhibited amyloid-β induced inflammatory damage in C6 glial cells. Arch Med Res. 2014; 45:152–157. PMID: 24480731.
Article27. Lee M, You HJ, Cho SH, Woo CH, Yoo MH, Joe EH, Kim JH. Implication of the small GTPase Rac1 in the generation of reactive oxygen species in response to β-amyloid in C6 astroglioma cells. Biochem J. 2002; 366:937–943. PMID: 12038964.
Article28. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–142. PMID: 1852778.29. Balez R, Ooi L. Getting to NO Alzheimer's disease: neuroprotection versus neurotoxicity mediated by nitric oxide. Oxid Med Cell Longev. 2016; 2016:3806157. PMID: 26697132.
Article30. Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and β-amyloid (25–35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic Biol Med. 2004; 37:325–338. PMID: 15223066.
Article31. Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. Peoniflorin attentuates Abeta((1–42))-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci. 2009; 280:71–78. PMID: 19268972.32. Lee AY, Lee MH, Lee S, Cho EJ. Neuroprotective effect of alpha-linolenic acid against Aβ-mediated inflammatory responses in C6 glial cell. J Agric Food Chem. 2018; 66:4853–4861. PMID: 29668263.
Article33. Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer's disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998; 8:65–72. PMID: 9458167.
Article34. Akama KT, Van Eldik LJ. β-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1β- and tumor necrosis factor-α (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000; 275:7918–7924. PMID: 10713108.35. Wang HM, Zhang T, Huang JK, Sun XJ. 3-N-butylphthalide (NBP) attenuates the amyloid-β-induced inflammatory responses in cultured astrocytes via the nuclear factor-κB signaling pathway. Cell Physiol Biochem. 2013; 32:235–242. PMID: 23899885.
Article36. Nam KN, Yae CG, Hong JW, Cho DH, Lee JH, Lee EH. Paeoniflorin, a monoterpene glycoside, attenuates lipopolysaccharide-induced neuronal injury and brain microglial inflammatory response. Biotechnol Lett. 2013; 35:1183–1189. PMID: 23559368.
Article37. Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G. Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses. PLoS One. 2012; 7:e49701. PMID: 23166749.
Article38. Reis PA, de Albuquerque CFG, Gutierrez TM, Silva AR, de Castro Faria Neto HC. Role of nitric oxide synthase in the function of the central nervous system under normal and infectious conditions. In : Saravi SSS, editor. Nitric Oxide Synthase - Simple Enzyme-Complex Roles. London: InTech;2017. p. 55–70.39. Jung CH, Kim JH, Park S, Kweon DH, Kim SH, Ko SG. Inhibitory effect of Agrimonia pilosa Ledeb. on inflammation by suppression of iNOS and ROS production. Immunol Invest. 2010; 39:159–170. PMID: 20136621.40. Tsoyi K, Ha YM, Kim YM, Lee YS, Kim HJ, Kim HJ, Seo HG, Lee JH, Chang KC. Activation of PPAR-γ by carbon monoxide from CORM-2 leads to the inhibition of iNOS but not COX-2 expression in LPS-stimulated macrophages. Inflammation. 2009; 32:364–371. PMID: 19705266.
Article41. Hensley K. Neuroinflammation in Alzheimer's disease: mechanisms, pathologic consequences, and potential for therapeutic manipulation. J Alzheimers Dis. 2010; 21:1–14. PMID: 20182045.
Article42. Kim MJ, Seong AR, Yoo JY, Jin CH, Lee YH, Kim YJ, Lee J, Jun WJ, Yoon HG. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol Nutr Food Res. 2011; 55:1798–1808. PMID: 22038937.
Article43. Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM. Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging. 2005; 26:645–654. PMID: 15708439.44. Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003; 40:1087–1093. PMID: 14687544.
Article45. Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo . Proc Natl Acad Sci U S A. 2003; 100:4162–4167. PMID: 12634421.46. Wang B, Dai W, Shi L, Teng H, Li X, Wang J, Geng W. Neuroprotection by paeoniflorin against nuclear factor kappa B-induced neuroinflammation on spinal cord injury. BioMed Res Int. 2018; 2018:9865403. PMID: 30627586.
Article47. Liu J, Jin DZ, Xiao L, Zhu XZ. Paeoniflorin attenuates chronic cerebral hypoperfusion-induced learning dysfunction and brain damage in rats. Brain Res. 2006; 1089:162–170. PMID: 16678139.
Article48. Zhang HR, Peng JH, Cheng XB, Shi BZ, Zhang MY, Xu RX. Paeoniflorin atttenuates amyloidogenesis and the inflammatory responses in a transgenic mouse model of Alzheimer's disease. Neurochem Res. 2015; 40:1583–1592. PMID: 26068144.
Article49. Kong Y, Peng Q, Lv N, Yuan J, Deng Z, Liang X, Chen S, Wang L. Paeoniflorin exerts neuroprotective effects in a transgenic mouse model of Alzheimer's disease via activation of adenosine A1 receptor. Neurosci Lett. 2020; 730:135016. PMID: 32371159.50. Liu H, Wang J, Wang J, Wang P, Xue Y. Paeoniflorin attenuates Aβ1–42-induced inflammation and chemotaxis of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling pathways. Brain Res. 2015; 1618:149–158. PMID: 26049130.51. He X, Xing D, Ding Y, Li Y, Xiang L, Wang W, Du L. Determination of paeoniflorin in rat hippocampus by high-performance liquid chromatography after intravenous administration of Paeoniae Radix extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2004; 802:277–281.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Treadmill Running Improves Spatial Learning Memory Through Inactivation of Nuclear Factor Kappa B/Mitogen-Activated Protein Kinase Signaling Pathway in Amyloid-β-Induced Alzheimer Disease Rats
- Paeoniflorin ameliorates neuropathic pain-induced depression-like behaviors in mice by inhibiting hippocampal neuroinflammation activated via TLR4/NF-kB pathway
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Paeoniflorin treatment regulates TLR4/NF-κB signaling, reduces cerebral oxidative stress and improves white matter integrity in neonatal hypoxic brain injury