Ann Rehabil Med.
2020 Oct;44(5):343-352. 10.5535/arm.19202.
Sequential Activation of AMPA Receptors and Glial Cells in a Pain Model of Lumbar Spine Disc Herniation
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, Yeungnam University College of Medicine, Daegu, Korea
- KMID: 2508459
- DOI: http://doi.org/10.5535/arm.19202
Abstract
Objective
To investigate the glial cell and AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor activity after surgery for disc herniation pain model.
Methods
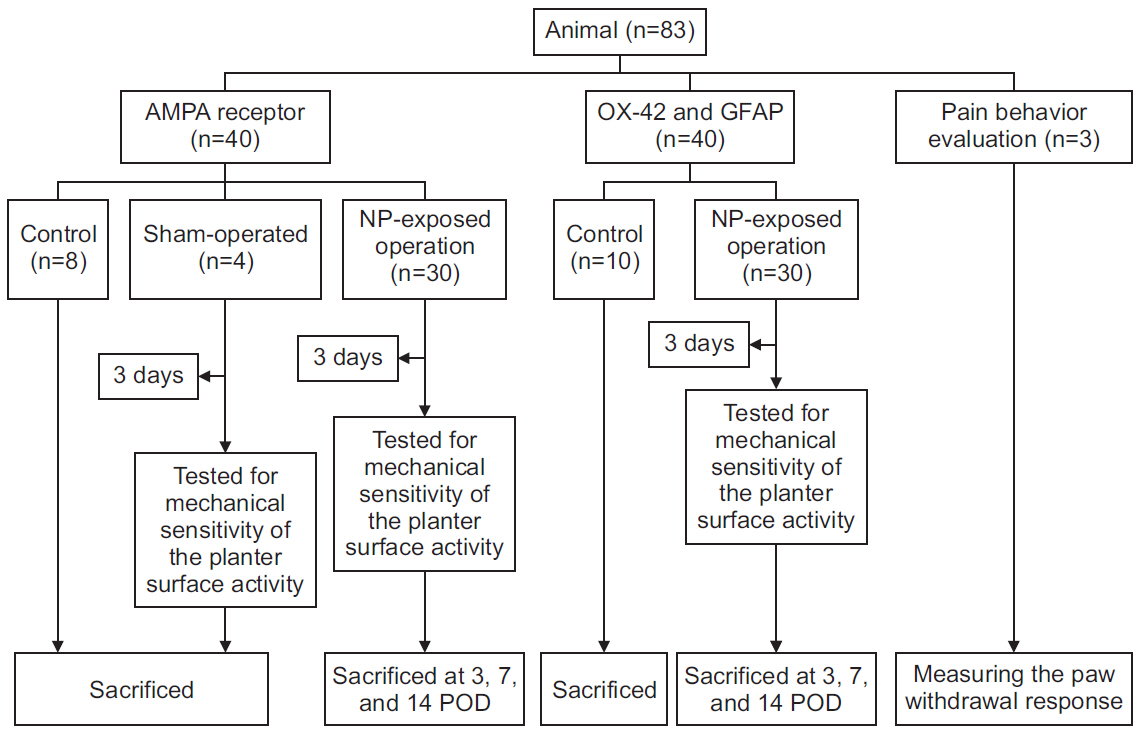
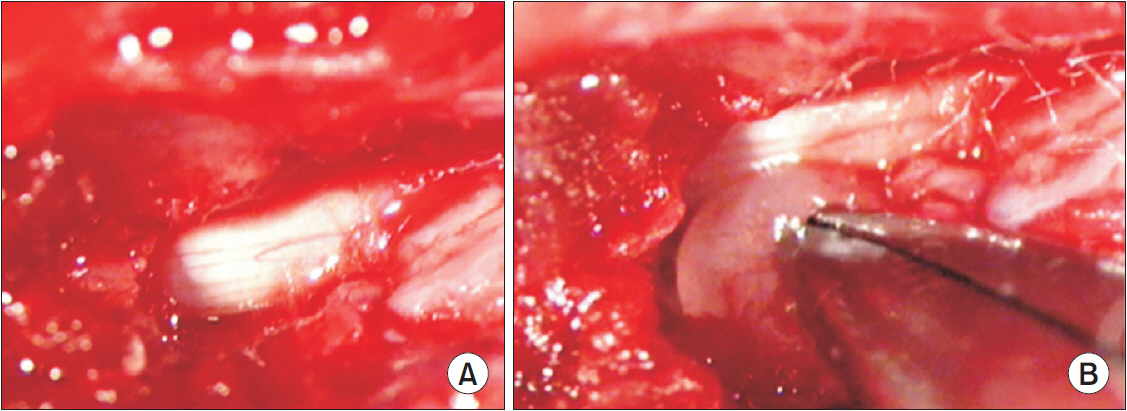
In total, 83 Sprague-Dawley rats were randomly assigned to the following groups: control (n=16), sham-operated (n=4), rats for pain behavior evaluation (n=3), nucleus pulposus-exposed groups for AMPA receptors (n=30), and glial cell (n=30). The rats were tested for mechanical allodynia; immunohistochemical staining for AMPA receptors (GluA1 and GluA2) and glial cells (OX-42 and glial fibrillary acid protein [GFAP]) in the spinal dorsal horn was performed on postoperative days 3, 7, and 14.
Results
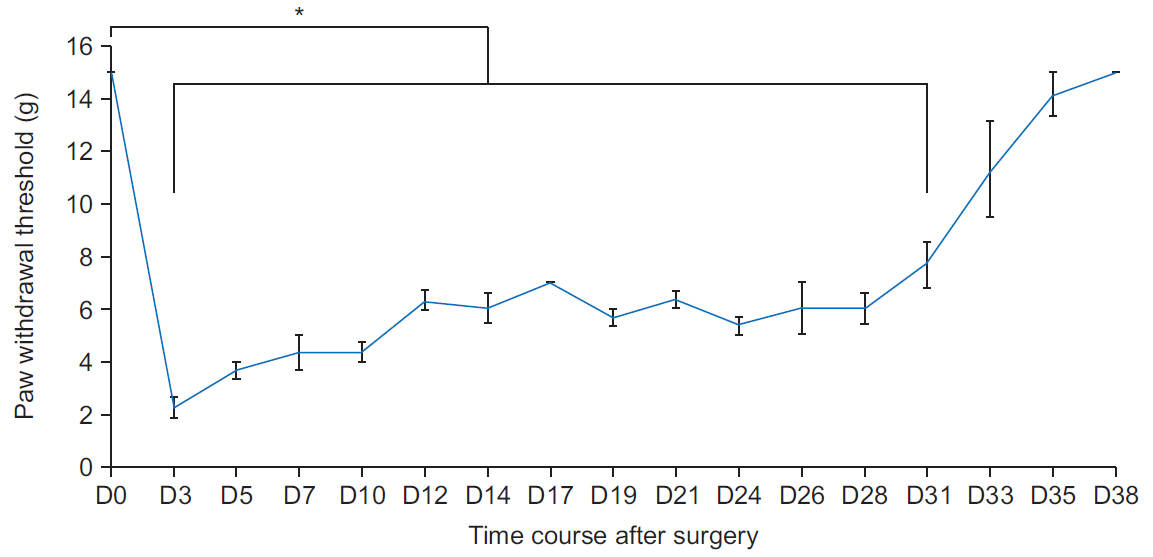
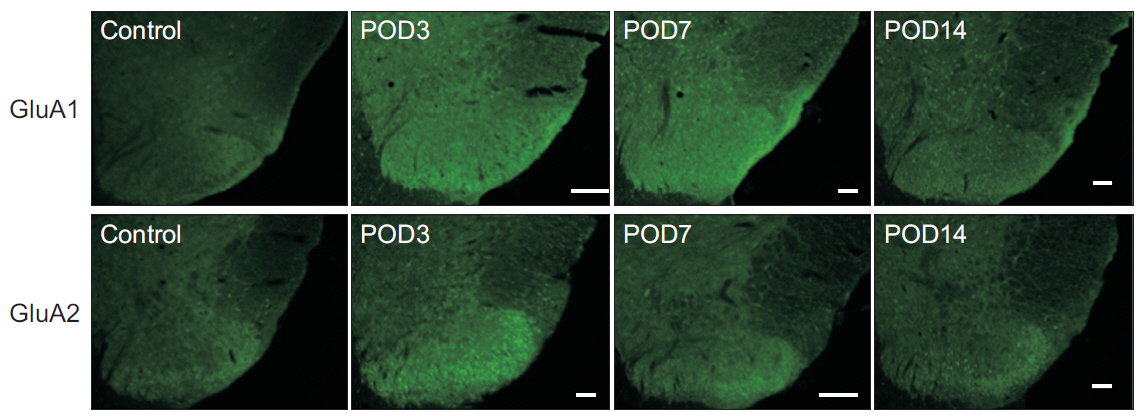
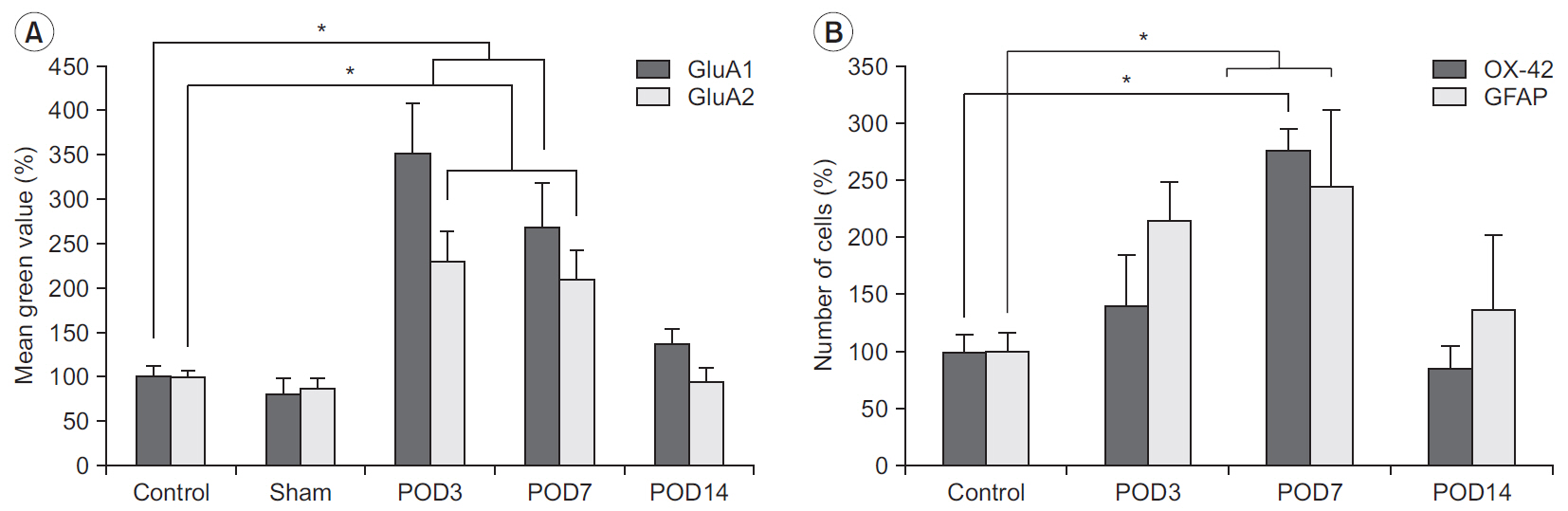
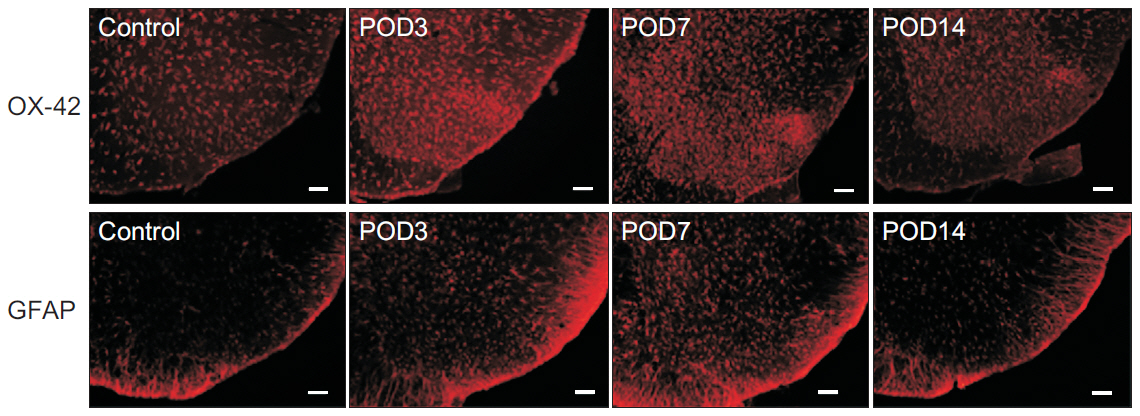
Mechanical withdrawal thresholds decreased after surgery, and this effect was maintained for up to 14 days. Immunohistochemical expression of GluA1 and GluA2 in the spinal dorsal horn had increased quantitatively on postoperative days 3 and 7 (p<0.05) to levels similar to that of the controls on postoperative day 14. Moreover, immunohistochemical expression of OX-42 and GFAP showed similar changes to AMPA receptors after surgery. Although the activity of AMPA receptors and glial cells achieved normalcy, the mechanical withdrawal threshold of the hind paw remained decreased 38 days after surgery.
Conclusion
The rat model of lumbar disc herniation showed increased expression of AMPA receptor and glial cell activity in the spinal dorsal horn 3 and 7 days after surgery, which deceased to control levels at 14 days. The AMPA receptors and glial cell activations showed similar patterns after disc herniation surgery.
Keyword
Figure
Reference
-
1. Garry EM, Fleetwood-Walker SM. A new view on how AMPA receptors and their interacting proteins mediate neuropathic pain. Pain. 2004; 109:210–3.
Article2. Kopach O, Voitenko N. Extrasynaptic AMPA receptors in the dorsal horn: evidence and functional significance. Brain Res Bull. 2013; 93:47–56.
Article3. Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, et al. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009; 29:3206–19.
Article4. Kopach O, Kao SC, Petralia RS, Belan P, Tao YX, Voitenko N. Inflammation alters trafficking of extrasynaptic AMPA receptors in tonically firing lamina II neurons of the rat spinal dorsal horn. Pain. 2011; 152:912–23.
Article5. Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig. 2016; 7:17–26.
Article6. Taves S, Berta T, Chen G, Ji RR. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast. 2013; 2013:753656.
Article7. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009; 10:23–36.
Article8. Mulleman D, Mammou S, Griffoul I, Watier H, Goupille P. Pathophysiology of disk-related sciatica. I.--Evidence supporting a chemical component. Joint Bone Spine. 2006; 73:151–8.
Article9. Sasaki N, Sekiguchi M, Shishido H, Kikuchi S, Yabuki S, Konno S. A comparison of pain-related behavior following local application of nucleus pulposus and/or mechanical compression on the dorsal root ganglion. Fukushima J Med Sci. 2011; 57:46–53.
Article10. Tachihara H, Sekiguchi M, Kikuchi S, Konno S. Do corticosteroids produce additional benefit in nerve root infiltration for lumbar disc herniation? Spine (Phila Pa 1976). 2008; 33:743–7.
Article11. Uesugi K, Sekiguchi M, Kikuchi S, Konno S. The effect of repeated restraint stress in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Eur Spine J. 2011; 20:1885–91.
Article12. Park HW, Ahn SH, Son JY, Kim SJ, Hwang SJ, Cho YW, et al. Pulsed radiofrequency application reduced mechanical hypersensitivity and microglial expression in neuropathic pain model. Pain Med. 2012; 13:1227–34.
Article13. Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010; 16:1248–57.
Article14. Cho JH, Lee DG. Translocation of AMPA receptors in the dorsal horn of the spinal cord corresponding to long-term depression following pulsed radiofrequency stimulation at the dorsal root ganglion. Pain Med. 2019; pnz307.
Article15. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009; 139:267–84.
Article16. Engelman HS, Allen TB, MacDermott AB. The distribution of neurons expressing calcium-permeable AMPA receptors in the superficial laminae of the spinal cord dorsal horn. J Neurosci. 1999; 19:2081–9.
Article17. Wigerblad G, Huie JR, Yin HZ, Leinders M, Pritchard RA, Koehrn FJ, et al. Inflammation-induced GluA1 trafficking and membrane insertion of Ca2+ permeable AMPA receptors in dorsal horn neurons is dependent on spinal tumor necrosis factor, PI3 kinase and protein kinase A. Exp Neurol. 2017; 293:144–58.18. Kim KJ, Lee SH, Hwang SJ, Oh SJ. Expression of pERK in the neurons and microglia in the spinal cord of the rat with neuropathic pain. J Korean Neurotraumatol Soc. 2005; 1:19–29.
Article19. Buenaventura RM, Datta S, Abdi S, Smith HS. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009; 12:233–51.20. Gong K, Kung LH, Magni G, Bhargava A, Jasmin L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One. 2014; 9:e95491.
Article21. Youn DH, Gerber G, Sather WA. Ionotropic glutamate receptors and voltage-gated Ca2+ channels in long-term potentiation of spinal dorsal horn synapses and pain hypersensitivity. Neural Plast. 2013; 2013:654257.22. Leem JW, Kim HK, Hulsebosch CE, Gwak YS. Ionotropic glutamate receptors contribute to maintained neuronal hyperexcitability following spinal cord injury in rats. Exp Neurol. 2010; 224:321–4.
Article23. Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal-glial transmission. J Anat. 2007; 210:651–60.
Article24. Wang Y, Wu J, Wu Z, Lin Q, Yue Y, Fang L. Regulation of AMPA receptors in spinal nociception. Mol Pain. 2010; 6:5.
Article25. Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003; 23:1750–8.
Article26. Chen SR, Zhou HY, Byun HS, Pan HL. Nerve injury increases GluA2-lacking AMPA receptor prevalence in spinal cords: functional significance and signaling mechanisms. J Pharmacol Exp Ther. 2013; 347:765–72.
Article27. Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, et al. Role of spinal cord alpha-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid receptors in complete Freund’s adjuvant-induced inflammatory pain. Mol Pain. 2008; 4:67.
Article28. Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003; 97:1108–16.
Article29. Tao YX. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology. 2010; 112:1259–65.30. Elward K, Gasque P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003; 40:85–94.
Article31. Lopez-Bayghen E, Espinoza-Rojo M, Ortega A. Glutamate down-regulates GLAST expression through AMPA receptors in Bergmann glial cells. Brain Res Mol Brain Res. 2003; 115:1–9.32. Sivakumar V, Ling EA, Lu J, Kaur C. Role of glutamate and its receptors and insulin-like growth factors in hypoxia induced periventricular white matter injury. Glia. 2010; 58:507–23.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid Repeated Recurrent Lumbar Disc Herniation after Microscopic Discectomies
- Lumbar Disc Screening Using Back Pain Questionnaires: Oswestry Low Back Pain Score, Aberdeen Low Back Pain Scale, and Acute Low Back Pain Screening Questionnaire
- Natural History and Clinical Manifestations of Lumbar Disc Herniation
- Classification and Imaging Study of the Lumbar Disc Herniation
- Redundant Nerve Roots of Cauda Equina Mimicking Intradural Disc Herniation: A Case Report