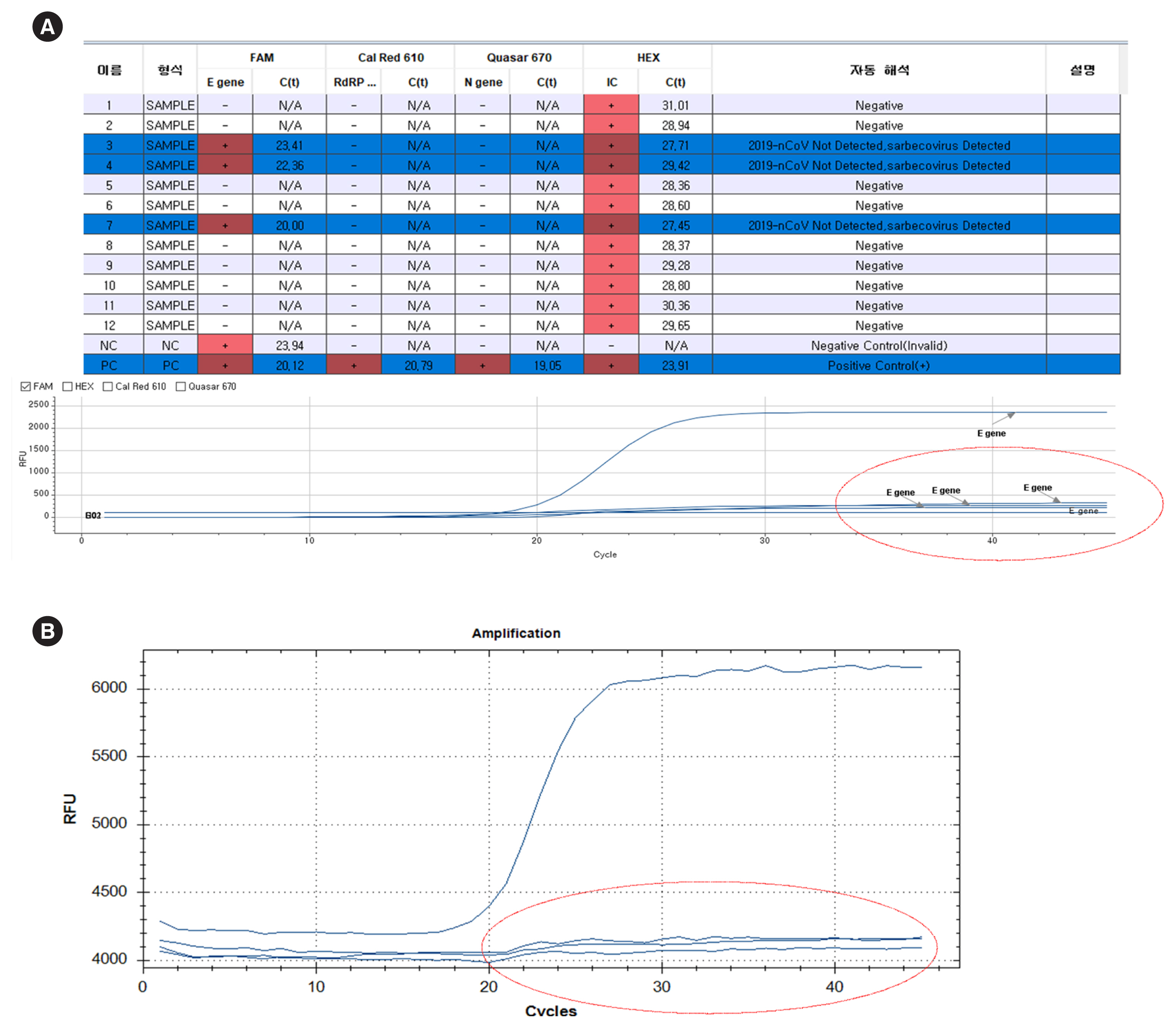
COVID-19 Molecular Testing in Korea: Practical Essentials and Answers From Experts Based on Experiences of Emergency Use Authorization Assays
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 2Department of Laboratory Medicine, National Health Insurance Service, Ilsan Hospital, Goyang, Korea
- 3Department of Laboratory Medicine, Seoul Medical Center, Seoul, Korea
- 4Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea
- 5Department of Laboratory Medicine, Keimyung University School of Medicine, Daegu, Korea
- 6Department of Laboratory Medicine, Hallym University College of Medicine, Chuncheon, Korea
- 7Department of Laboratory Medicine, Jeonbuk National University Medical School and Hospital, Jeonju, Korea
- 8Department of Laboratory Medicine, National Medical Center, Seoul, Korea
- 9Department of Laboratory Medicine, Kangwon National University School of Medicine, Chuncheon, Korea
- 10Center for Laboratory Control of Infectious Diseases, Korea Centers for Disease Control and Prevention, Osong, Korea
- 11Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2507789
- DOI: http://doi.org/10.3343/alm.2020.40.6.439
Figure
Cited by 10 articles
-
Role of Genetic Variants and Gene Expression in the Susceptibility and Severity of COVID-19
Sarita Choudhary, Karli Sreenivasulu, Prasenjit Mitra, Sanjeev Misra, Praveen Sharma
Ann Lab Med. 2021;41(2):129-138. doi: 10.3343/alm.2021.41.2.129.Surveillance of Coronavirus Disease 2019 (COVID-19) Testing in Clinical Laboratories in Korea
Hee Jae Huh, Ki Ho Hong, Taek Soo Kim, Sang Hoon Song, Kyoung Ho Roh, Hyukmin Lee, Gye Cheol Kwon
Ann Lab Med. 2021;41(2):225-229. doi: 10.3343/alm.2021.41.2.225.Laboratory Diagnosis of COVID-19 in Korea
Kyunghoon Lee
Ewha Med J. 2021;44(1):1-10. doi: 10.12771/emj.2021.44.1.1.Response of Clinical Laboratories to the Ongoing COVID-19 Pandemic
Young Jin Kim, Heungsup Sung, Chang-Seok Ki, Mina Hur
Ann Lab Med. 2021;41(6):519-520. doi: 10.3343/alm.2021.41.6.519.Early Laboratory Preparedness of the Korea Disease Control and Prevention Agency and Response to Unknown Pneumonia Outbreak from Wuhan, China, in January 2020
Il-Hwan Kim, Byung-Hak Kang, Seung Hee Seo, Ye Eun Park, Gab Jung Kim, Sang Won Lee, Jun Hyeong Jang, Su Kyoung Jo, Jun Ho Jeon, Jeong-Min Kim, Yoon-Seok Chung, Myung-Guk Han, Sang-Oun Jung, Junyoung Kim, Kyu-Jam Hwang, Cheon-Kwon Yoo, Gi-eun Rhie
Ann Lab Med. 2021;41(6):532-539. doi: 10.3343/alm.2021.41.6.532.Quantum Leap and Future Contribution of Annals of Laboratory Medicine
Young Jin Kim, Mina Hur
Ann Lab Med. 2022;42(1):1-2. doi: 10.3343/alm.2022.42.1.1.Rates of Coinfection Between SARS-CoV-2 and Other Respiratory Viruses in Korea
Young-gon Kim, Hyunwoong Park, So Yeon Kim, Ki Ho Hong, Man Jin Kim, Jee-Soo Lee, Sung-Sup Park, Moon-Woo Seong
Ann Lab Med. 2022;42(1):110-112. doi: 10.3343/alm.2022.42.1.110.Clinical Evaluation of the Rapid STANDARD Q COVID-19 Ag Test for the Screening of Severe Acute Respiratory Syndrome Coronavirus 2
Hyung Woo Kim, Mikyoung Park, Jong Ho Lee
Ann Lab Med. 2022;42(1):100-104. doi: 10.3343/alm.2022.42.1.100.Update of Guidelines for Laboratory Diagnosis of COVID-19 in Korea
Ki Ho Hong, Gab Jung Kim, Kyoung Ho Roh, Heungsup Sung, Jaehyeon Lee, So Yeon Kim, Taek Soo Kim, Jae-Sun Park, Hee Jae Huh, Younhee Park, Jae-Seok Kim, Hyun Soo Kim, Moon-Woo Seong, Nam Hee Ryoo, Sang Hoon Song, Hyukmin Lee, Gye Cheol Kwon, Cheon Kwon Yoo
Ann Lab Med. 2022;42(4):391-397. doi: 10.3343/alm.2022.42.4.391.Evaluation of the AdvanSure One-Stop COVID-19 Plus Kit for SARS-CoV-2 Detection Using a Streamlined RNA Extraction Method
Tae Yeul Kim, Hyang Jin Shim, Eunjung Jeong, Minhee Kang, Ja-Hyun Jang, Hee Jae Huh, Nam Yong Lee
Ann Lab Med. 2023;43(5):508-511. doi: 10.3343/alm.2023.43.5.508.
Reference
-
1. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (Updated on February 11, 2020).2. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (Updated on March 11, 2020).3. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020; 40:351–60.
Article4. Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html (Updated on April 14, 2020).5. World Health Organization. Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance (Updated on March 2, 2020).6. Wang X, Tan L, Wang X, Liu W, Lu Y, Cheng L, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020; 94:107–9.
Article7. Sung H, Yong D, Ki CS, Kim JS, Seong MW, Lee H, et al. Comparative evaluation of three homogenization methods for isolating Middle East respiratory syndrome coronavirus nucleic acids from sputum samples for real-time reverse transcription PCR. Ann Lab Med. 2016; 36:457–62.
Article8. Pan Y, Long L, Zhang D, Yan T, Cui S, Yang P, et al. Potential false-negative nucleic acid testing results for severe acute respiratory syndrome coronavirus 2 from thermal inactivation of samples with low viral loads. Clin Chem. 2020; Apr. 4. pii: hvaa091. DOI: 10.1093/clinchem/hvaa091.
Article9. Yen-Lieberman B, Brambilla D, Jackson B, Bremer J, Coombs R, Cronin M, et al. Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J Clin Microbiol. 1996; 34:2695–701.
Article10. Kim S, Park SJ, Namgoong S, Sung H, Kim MN. Comparative evaluation of two automated systems for nucleic acid extraction of BK virus: NucliSens easyMAG versus BioRobot MDx. J Virol Methods. 2009; 162:208–12.
Article11. COVID-19 Diagnosis Test Management Committee. Q&A for COVID-19 testings. http://www.kslm.org/ (Updated on March 24, 2020).12. Sung H, Yoo CK, Han MG, Lee SW, Lee H, Chun S, et al. Preparedness and rapid implementation of external quality assessment helped quickly increase COVID-19 testing capacity in the Republic of Korea. Clin Chem. 2020; Apr. 22. pii: hvaa097. DOI: 10.1093/clinchem/hvaa097.
Article13. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; 25:2000045.
Article14. CLSI. Establishing molecular testing in clinical laboratory environments; approved guideline. CLSI MM19-A. Wayne, PA: Clinical and Laboratory Standards Institute;2011.15. Park GW, Chhabra P, Vinjé J. Swab sampling method for the detection of human norovirus on surface. J Vis Exp. 2017; 120:55205.16. Thermo Fisher Scientific. Distinguishing-real-signal-from-background-noise-ask-taqman-41. https://www.thermofisher.com/blog/behindthebench/ (Updated on December 23, 2016).17. Korea Centers for Disease Control and Prevention. Management guidelines for coronavirus disease-19. Version 8. http://ncov.mohw.go.kr/duBoardList.do?brdId=2&brdGubun=28 (Updated on May 11, 2020).18. Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with suspected or confirmed COVID-19 (Interim Guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html#f1 (Updated on April 30, 2020).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: COVID-19 Molecular Testing in Korea: Practical Essentials and Answers From Experts Based on Experiences of Emergency Use Authorization Assays
- The Work Experiences of Emergency Room Nurses during the COVID-19 Pandemic
- Decision on Chemotherapy amidst COVID-19 Pandemic: A Review and a Practical Approach from Iran
- COVID-19 Antiviral and Treatment Candidates: Current Status
- Clinical Practice Experience of Nursing Students in the Context of the COVID-19 Pandemic