Ann Pediatr Endocrinol Metab.
2020 Sep;25(3):174-181. 10.6065/apem.1938174.087.
Adipokines in young survivors of childhood acute lymphocytic leukemia revisited: beyond fat mass
- Affiliations
-
- 1Division of Pediatric Endocrinology, Federal University of Sao Paulo - UNIFESP/EPM, Sao Paulo, Brazil
- 2Pediatric Oncology Institute - IOP/GRAACC, Sao Paulo, Brazil
- 3Division of Biostatistics, Department of Preventive Medicine, UNIFESP/EPM, Sao Paulo, Brazil
- 4Division of Pediatric Oncology - Hospital Santa Marcelina/TUCCA, Sao Paulo, Brazil
- 5Laboratory of Investigation on Metabolism and Diabetes - LIMED, Faculty of Medical Sciences, State University of Campinas - UNICAMP, Campinas, Brazil
- 6Division of Pediatric Endocrinology, Faculty of Medical Sciences, UNICAMP, Campinas, Brazil
- KMID: 2507560
- DOI: http://doi.org/10.6065/apem.1938174.087
Abstract
- Purpose
This cross-sectional study evaluated the relationship between adipokines (leptin, adiponectin, visfatin, and resistin) and adiposity indexes regarding sex and cranial radiotherapy exposure among young acute lymphocytic leukemia survivors.
Methods
A multivariate analysis of covariance (MANCOVA) was used to evaluate the joint effect of sex, cranial radiotherapy, and body mass index (BMI) z-score (model 1) or fat mass index (FMI) (model 2) on adipokines.
Results
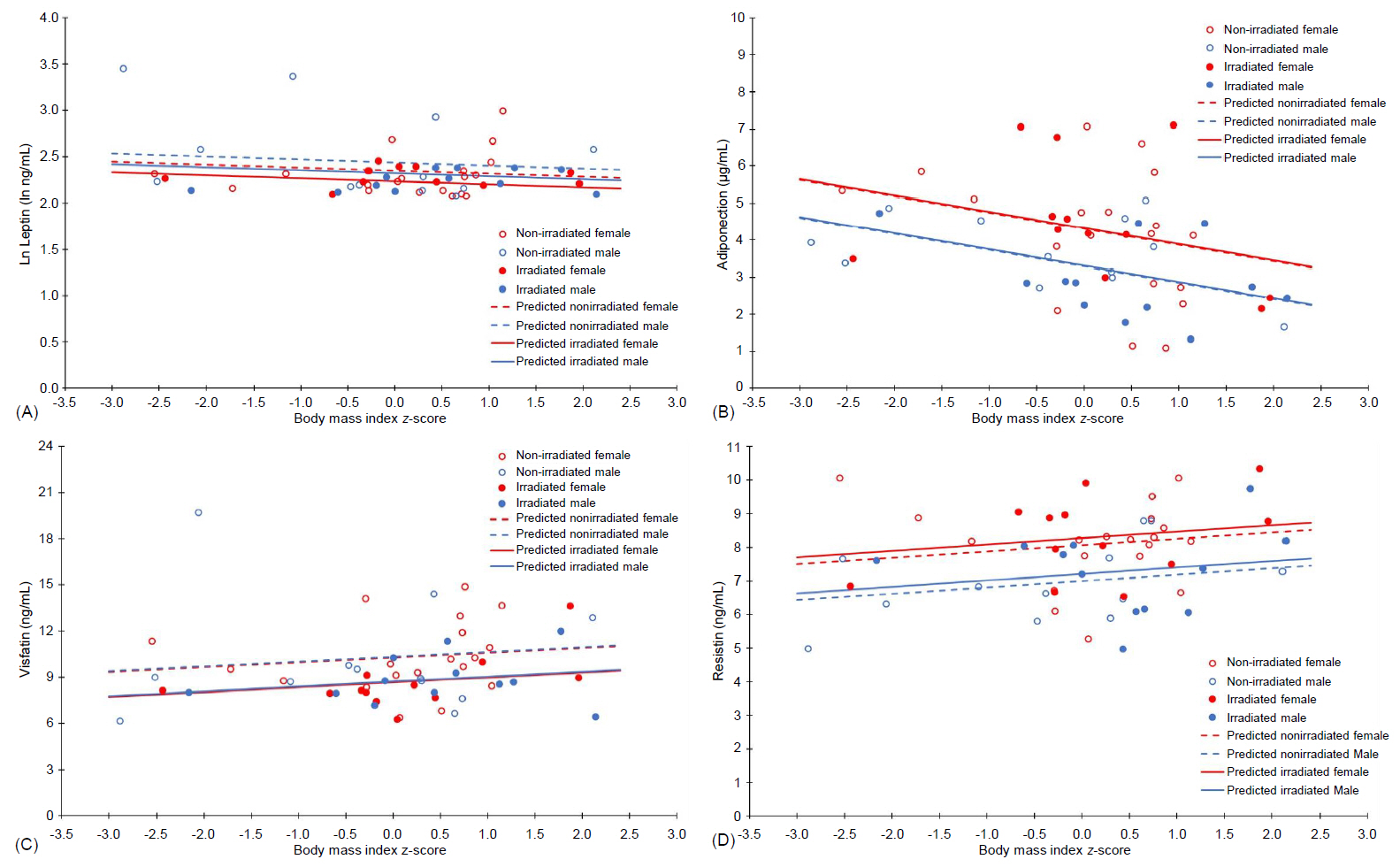
This study included 55 survivors of childhood acute lymphocytic leukemia between 15 and 23 years of age from both sexes (56.4% female); 43.6% of the sample had undergone cranial radiotherapy (18–24 Gy). The BMI z-score, the FMI, and sex (P<0.050 for all) influenced at least one adipokine, while cranial radiotherapy exposure was marginal in model 2. Parameter estimates from the MANCOVA's final model showed that the BMI z-score (β=-0.437, P=0.010) and the FMI (β=-0.209, P=0.004) negatively influenced adiponectin, while the FMI positively affected resistin (β=0.142, P=0.020). The relationship between leptin, visfatin, and the adiposity ndexes could not be established. In model 1, females presented with increased adiponectin (β=-1.014, P=0.011) and resistin (β=-1.067, P=0.002) levels; in model 2, female sex positively affected adiponectin (β=-1.515, P=0.001) and marginally influenced resistin (β=-0.707, P=0.054) levels. Cranial radiotherapy negatively determined visfatin levels in both final models (P<0.050).
Conclusion
Changes in body fat may be associated with adipose tissue dysfunction and should be carefully evaluated in survivors of acute lymphocytic leukemia, considering both sex and cranial radiotherapy exposure, to treat disorders that may possibly aggravate their risk for early cardiovascular disease.
Keyword
Figure
Reference
-
References
1. Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL). Eur J Cancer Care (Engl). 2005; 14:53–62.
Article2. Link K, Moëll C, Garwicz S, Cavallin-Ståhl E, Björk J, Thilén U, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 2004; 89:5003–12.
Article3. Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006; 114:2710–38.
Article4. Siviero-Miachon AA, Spinola-Castro AM, Guerra-Junior G. Detection of metabolic syndrome features among childhood cancer survivors: a target to prevent disease. Vasc Health Risk Manag. 2008; 4:825–36.5. Siviero-Miachon AA, Spinola-Castro AM, Guerra-Junior G. Adiposity in childhood cancer survivors: insights into obesity physiopathology. Arq Bras Endocrinol Metab. 2009; 53:190–200.
Article6. Siviero-Miachon AA, Spinola-Castro AM, Lee ML, Andreoni S, Geloneze B, Lederman H, et al. Cranial radiotherapy predisposes to abdominal adiposity in survivors of childhood acute lymphocytic leukemia. Radiat Oncol. 2013; 8:39.
Article7. van Waas M, Neggers SJ, Pieters R, van den Heuvel-Eibrink MM. Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann Oncol. 2010; 21:1121–6.
Article8. Oeffinger KC, Buchanan GR, Eshelman DA, Denke MA, Andrews TC, Germak JA, et al. Cardiovascular risk factors in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2001; 23:424–30.
Article9. Janiszewski PM, Oeffinger KC, Church TS, Dunn AL, Eshelman DA, Victor RG, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007; 92:3816–21.
Article10. Spinola-Castro AM, Siviero-Miachon AA, Guerra-Junior G, Geloneze B. Insulin resistance in childhood cancer survivors: a link between metabolic syndrome features. In : Yao EB, editor. Insulin resistance: new research. New York: Nova Science Publishers;2009. p. 235–51.11. Siviero-Miachon AA, Spinola-Castro AM, Guerra-Junior G. Metabolic syndrome and cancer: cause or consequence? J Metabol Syndro. 2012; 1:e103.12. Barbosa-Cortés L, López-Alarcón M, Mejía-Aranguré JM, Klünder-Klünder M, Del Carmen Rodríguez-Zepeda M, Rivera-Márquez H, et al. Adipokines, insulin resistance, and adiposity as a predictors of metabolic syndrome in child survivors of lymphoma and acute lymphoblastic leukemia of a developing country. BMC Cancer. 2017; 17:125.
Article13. Aref S, Ibrahim L, Azmy E, Al Ashary R. Impact of serum adiponectin and leptin levels in acute leukemia. Hematology. 2013; 18:198–203.
Article14. Hino M, Nakao T, Yamane T, Ohta K, Takubo T, Tatsumi N. Leptin receptor and leukemia. Leuk Lymphoma. 2000; 36:457–61.
Article15. Hamed NA, Sharaki OA, Zeidan MM. Leptin in acute leukaemias: relationship to interleukin-6 and vascular endothelial growth factor. Egypt J Immunol. 2003; 10:57–66.16. Siviero-Miachon AA, Spinola-C astro AM, Tosta-Hernandez PD, de Martino Lee ML, Petrilli AS. Leptin assessment in acute lymphocytic leukemia survivors: role of cranial radiotherapy? J Pediatr Hematol Oncol. 2007; 29:776–82.
Article17. Moschovi M, Trimis G, Vounatsou M, Katsibardi K, Margeli A, Damianos A, et al. Serial plasma concentrations of adiponectin, leptin, and resistin during therapy in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010; 32:e8–13.
Article18. Kohler JA, Moon RJ, Wright S, Willows E, Davies JH. Increased adiposity and altered adipocyte function in female survivors of childhood acute lymphoblastic leukaemia treated without cranial radiation. Horm Res Paediatr. 2011; 75:433–40.
Article19. Bülow B, Link K, Ahrén B, Nilsson AS, Erfurth EM. Survivors of childhood acute lymphoblastic leukaemia, with radiation - induced GH deficiency, exhibit hyperleptinaemia and impaired insulin sensitivity, unaffected by 12 months of GH treatment. Clin Endocrinol (Oxf). 2004; 61:683–91.20. Tonorezos ES, Vega GL, Sklar CA, Chou JF, Moskowitz CS, Mo Q, et al. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatr Blood Cancer. 2012; 58:31–6.
Article21. Siviero-Miachon AA, Spinola-Castro AM, de Martino Lee ML, de Castro Monteiro CM, de Camargo Carvalho AC, Calixto AR, et al. Subcutaneous adipose tissue plays a beneficial effect on subclinical atherosclerosis in young survivors of acute lymphocytic leukemia. Vasc Health Risk Manag. 2015; 11:479–88.
Article22. Jahnukainen K, Heikkinen R, Henriksson M, Andersson S, Ivaska KK, Puukko-Viertomies LR, et al. Increased body adiposity and serum leptin concentrations in very long-term adult male survivors of childhood acute lymphoblastic leukemia. Horm Res Paediatr. 2015; 84:108–15.
Article23. Brennan BM, Rahim A, Blum WF, Adams JA, Eden OB, Shalet SM. Hyperleptinaemia in young adults following cranial irradiation in childhood: growth hormone deficiency or leptin insensitivity? Clin Endocrinol (Oxford). 1999; 50:163–9.
Article24. Karaman S, Ercan O, Yildiz I, Bolayirli M, Celkan T, Apak H, et al. Late effects of childhood ALL treatment on body mass index and serum leptin levels. J Pediatr Endocrinol Metab. 2010; 23:669–74.
Article25. Brandalise S, Viana M, Pereira W, Loggetto S, Zouain G, Lee M, et al. Chemotherapy in 853 unselected childhood ALL patients. Results of the Brazilian multicenter trial GBTLI ALL-93 [abstract]. Pediatr Blood Cancer. 2004; 43:s399.26. Scrideli CA, Assumpção JG, Ganazza MA, Araújo M, Toledo SR, Lee ML, et al. A simplified minimal residual disease polymerase chain reaction method at early treatment points can stratify children with acute lymphoblastic leukemia into good and poor outcome groups. Haematologica. 2009; 94:781–9.
Article27. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969; 44:291–303.
Article28. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970; 45:13–23.
Article29. Siviero-Miachon AA, Spinola-Castro AM, de Martino Lee ML, Calixto AR, Geloneze B, Lazaretti-Castro M, et al. Visfatin is a positive predictor of bone mineral density in young survivors of acute lymphocytic leukemia. J Bone Miner Metab. 2017; 35:73–82.
Article30. National Center for Health Statistics (2000). Clinical growth charts [Internet]. Centers for Disease Control and Prevention;[2019 Aug 1]. Available from: http://www.cdc.gov/growthcharts/clinical_charts.htm.31. Gui Y, Silha JV, Murphy LJ. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes Res. 2004; 12:1481–91.
Article32. Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Müller J, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997; 82:2904–10.
Article33. Brandão CM, Lombardi MT, Nishida SK, Hauache OM, Vieira JG. Serum leptin concentration during puberty in healthy nonobese adolescents. Braz J Med Biol Res. 2003; 36:1293–6.
Article34. Romacho T, Sánchez-Ferrer CF, Peiró C. Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm. 2013; 2013:946427.
Article35. Dozio E, Corsi MM, Ruscica M, Passafaro L, Steffani L, Banfi G, et al. Adipokine actions on cartilage homeostasis. Adv Clin Chem. 2011; 55:61–79.
Article36. Scotece M, Conde J, Gómez R, López V, Lago F, Gómez-Reino JJ, et al. Beyond fat mass: exploring the role of adipokines in rheumatic diseases. ScientificWorldJournal. 2011; 11:1932–47.
Article37. Skoczen S, Tomasik PJ, Gozdzik J, Fijorek K, Krasowska-Kwiecien A, Wiecha O, et al. Visfatin concentrations in children with leukemia before and after stem cell transplantation. Exp Hematol. 2014; 42:252–60.
Article38. Lang K, Ratke J. Leptin and adiponectin: new players in the field of tumor cell and leukocyte migration. Cell Commun Signal. 2009; 7:27.
Article39. Reilly JJ, Kelly A, Ness P, Dorosty AR, Wallace WH, Gibson BE, et al. Premature adiposity rebound in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001; 86:2775–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Observations on Childhood Acute Lymphocytic Leukemia
- Weight status in survivors of childhood acute lymphocytic leukemia in South Korea: a retrospective descriptive study
- A Case of Acute Lymphocytic Leukemia with Both Renal Enlargement
- Preleukemic State Preceding Acute Lymphocytic Leukemia in Childhood
- Acute Myeloid Leukemia after Chemotherapy for Osteosarcoma: A Case Report