Prog Med Phys.
2020 Sep;31(3):71-80. 10.14316/pmp.2020.31.3.71.
Carbon Ion Therapy: A Review of an Advanced Technology
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University Hospital, Seoul, Korea
- 2Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea
- 3Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- 4Robotics Research Laboratory for Extreme Environments, Advanced Institute of Convergence Technology, Suwon, Korea
- 5Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea
- 6Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2507480
- DOI: http://doi.org/10.14316/pmp.2020.31.3.71
Abstract
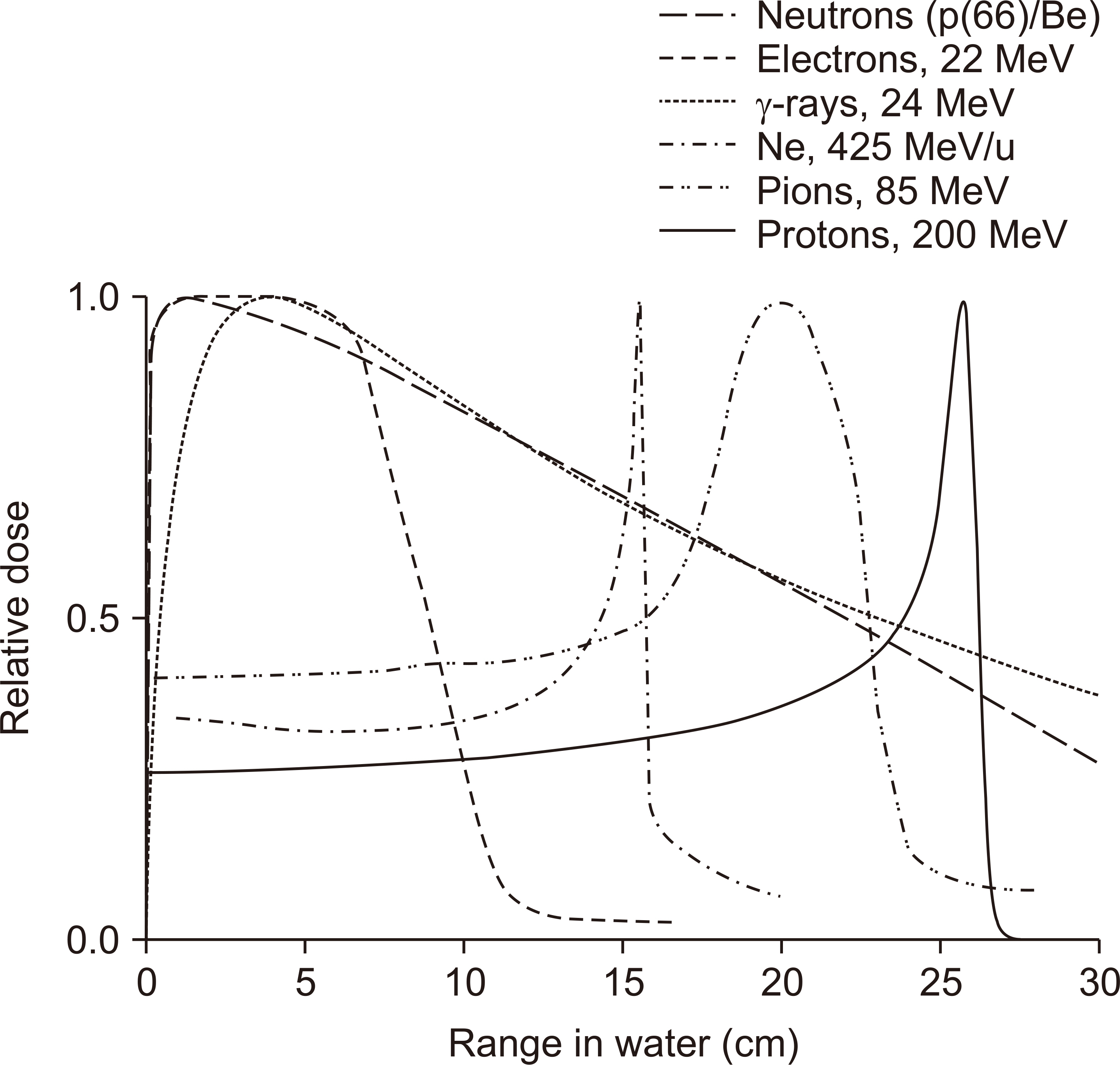
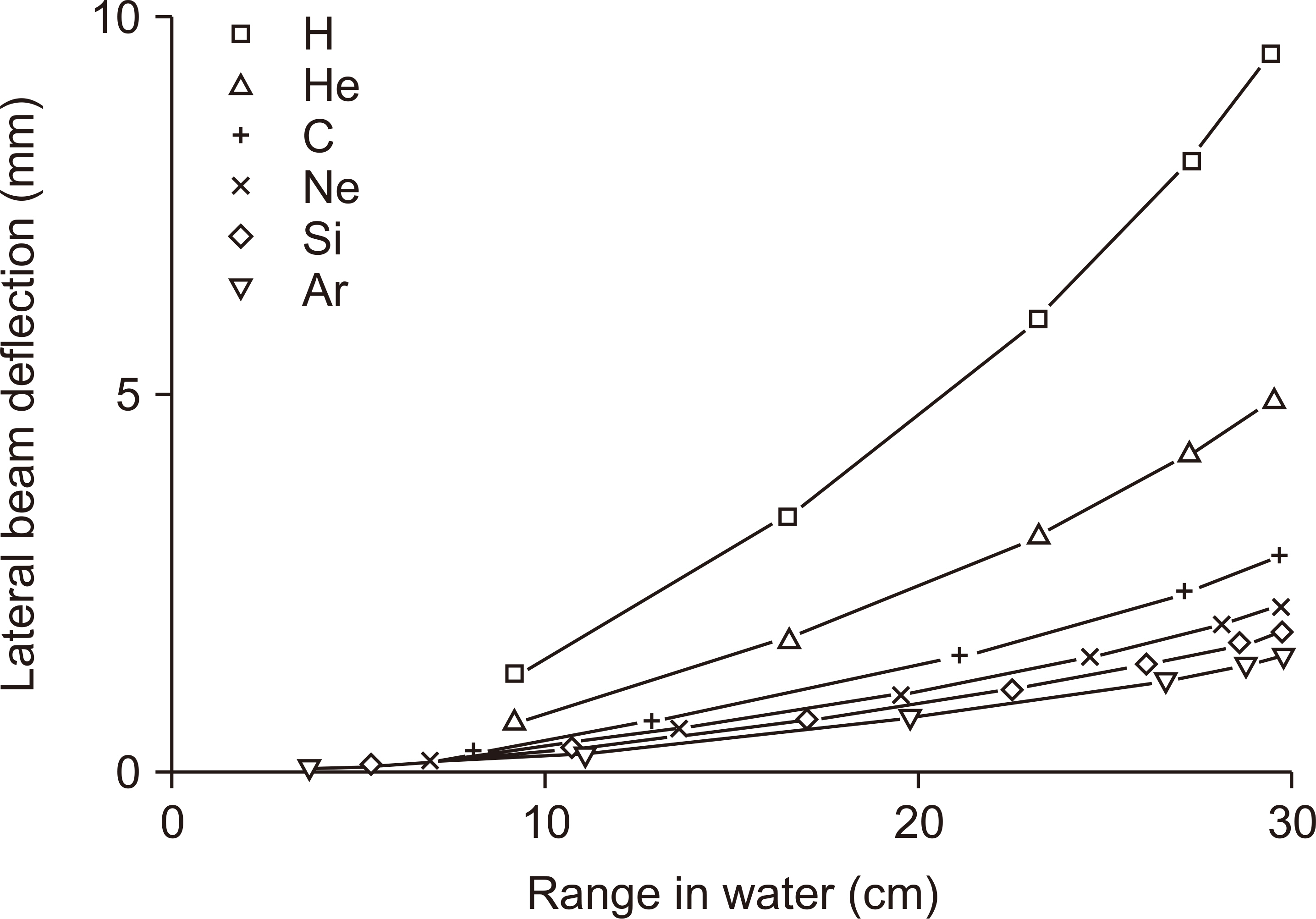
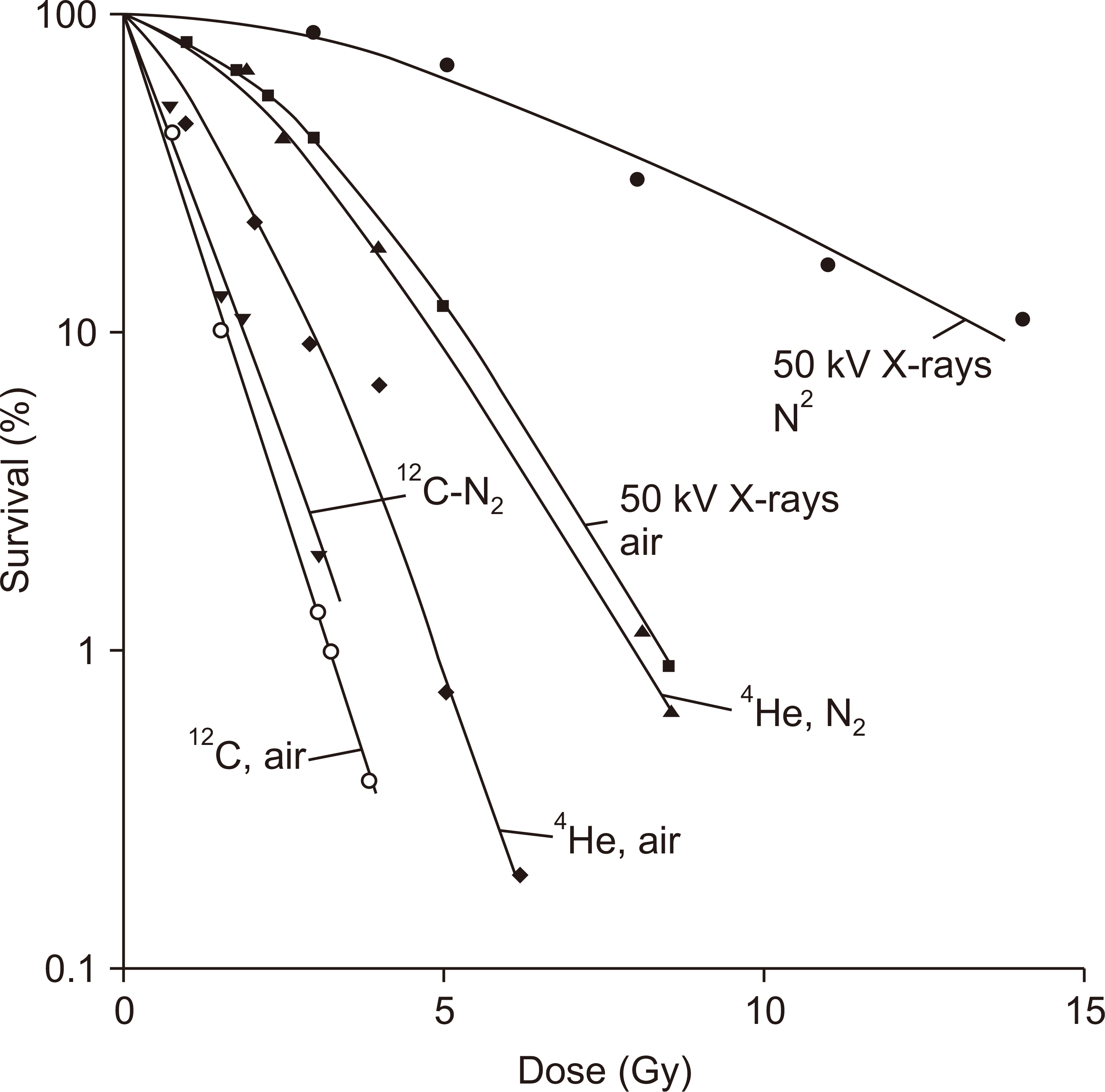
- This paper provides a brief review of the advanced technologies for carbon ion radiotherapy (CIRT), with a focus on current developments. Compared to photon beam therapy, treatment using heavy ions, especially a carbon beam, has potential advantages due to its physical and biological pro perties. Carbon ion beams with high linear energy transfer demonstrate high relative biological effectiveness in cell killing, particularly at the Bragg peak. With these unique properties, CIRT allows for accurate targeting and dose escalation for tumors with better sparing of adjacent normal tissues. Recently, the available CIRT technologies included fast pencil beam scanning, supercon ducting rotating gantry, respiratory motion management, and accurate beam modeling for the treatment planning system. These techniques provide precise treatment, operational efficiency, and patient comfort. Currently, there are 12 CIRT facilities worldwide; with technological improvements, they continue to grow in number. Ongoing technological developments include the use of multiple ion beams, effective beam delivery, accurate biological modeling, and downsizing the facility.
Keyword
Figure
Cited by 2 articles
-
Who Will Benefit from Charged-Particle Therapy?
Kyung Su Kim, Hong-Gyun Wu
Cancer Res Treat. 2021;53(3):621-634. doi: 10.4143/crt.2021.299.Technological Advances in Charged-Particle Therapy
Jong Min Park, Jung-in Kim, Hong-Gyun Wu
Cancer Res Treat. 2021;53(3):635-640. doi: 10.4143/crt.2021.706.
Reference
-
References
1. Wilson RR. 1946; Radiological use of fast protons. Radiology. 47:487–491. DOI: 10.1148/47.5.487. PMID: 20274616.
Article2. Lawrence JH, Tobias CA, Born JL, McCombs RK, Roberts JE, Anger HO, et al. 1958; Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res. 18:121–134. PMID: 13511365.3. Castro JR, Linstadt DE, Bahary JP, Petti PL, Daftari I, Collier JM, et al. 1994; Experience in charged particle irradiation of tumors of the skull base: 1977-1992. Int J Radiat Oncol Biol Phys. 29:647–655. DOI: 10.1016/0360-3016(94)90550-9. PMID: 8040010.
Article4. Tsujii H, Mizoe JE, Kamada T, Baba M, Kato S, Kato H, et al. 2004; Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother Oncol. 73 Suppl 2:S41–S49. DOI: 10.1016/S0167-8140(04)80012-4. PMID: 15971308.
Article5. Particle Therapy Co-Operative Group. 2018. Particle therapy facilities in clinical operation. Particle Therapy Co-Operative Group;Available from: https://www.ptcog.ch/index.php/facilities-in-operation . cited 2020 May 5.6. Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, et al. 2015; Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 16:e93–e100. DOI: 10.1016/S1470-2045(14)70412-7. PMID: 25638685.
Article7. Pommier P, Lievens Y, Feschet F, Borras JM, Baron MH, Shtiliyanova A, et al. 2010; Simulating demand for innovative radiotherapies: an illustrative model based on carbon ion and proton radiotherapy. Radiother Oncol. 96:243–249. DOI: 10.1016/j.radonc.2010.04.010. PMID: 20452693.
Article8. Combs SE, Ellerbrock M, Haberer T, Habermehl D, Hoess A, Jäkel O, et al. 2010; Heidelberg Ion Therapy Center (HIT): initial clinical experience in the first 80 patients. Acta Oncol. 49:1132–1140. DOI: 10.3109/0284186X.2010.498432. PMID: 20831505.
Article9. Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, et al. 1999; Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 44:201–210. DOI: 10.1016/S0360-3016(98)00544-6. PMID: 10219815.
Article10. Torikoshi M, Minohara S, Kanematsu N, Komori M, Kanazawa M, Noda K, et al. 2007; Irradiation system for HIMAC. J Radiat Res. 48 Suppl A:A15–A25. DOI: 10.1269/jrr.48.A15. PMID: 17513897.
Article11. Minohara S, Kanai T, Endo M, Noda K, Kanazawa M. 2000; Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys. 47:1097–1103. DOI: 10.1016/S0360-3016(00)00524-1. PMID: 10863083.
Article12. Haberer T, Becher W, Schardt D, Kraft G. 1993; Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods Phys Res Sect A. 330:296–305. DOI: 10.1016/0168-9002(93)91335-K.
Article13. Combs SE, Jäkel O, Haberer T, Debus J. 2010; Particle therapy at the Heidelberg Ion Therapy Center (HIT)- integrated research-driven university-hospital-based radiation oncology service in Heidelberg, Germany. Radiother Oncol. 95:41–44. DOI: 10.1016/j.radonc.2010.02.016. PMID: 20227124.14. Linz U. 2012. Physical and biological rationale for using Ions in therapy. Ion beam therapy. Springer;Berlin, Heidelberg: DOI: 10.1007/978-3-642-21414-1_4.
Article15. Chen GT, Castro JR, Quivey JM. 1981; Heavy charged particle radiotherapy. Annu Rev Biophys Bioeng. 10:499–529. DOI: 10.1146/annurev.bb.10.060181.002435. PMID: 7020583.
Article16. Kraft G, Kraft-Weyrather W, Ritter S, Scholz M, Stanton J. 1989; Cellular and subcellular effect of heavy ions: a comparison of the induction of strand breaks and chromosomal aberration with the incidence of inactivation and mutation. Adv Space Res. 9:59–72. DOI: 10.1016/0273-1177(89)90423-7. PMID: 11537316.
Article17. Chu W, Ludewigt B, Renner T. 1993; Instrumentation for treatment of cancer using proton and light-ion beams. Rev Sci Instrum. 64:2055–2122. DOI: 10.1063/1.1143946.
Article18. Todd P. 1968; Fractionated heavy ion irradiation of cultured human cells. Radiat Res. 34:378–389. DOI: 10.2307/3572563. PMID: 5653430.
Article19. Kanai T, Kanematsu N, Minohara S, Komori M, Torikoshi M, Asakura H, et al. 2006; Commissioning of a conformal irradiation system for heavy-ion radiotherapy using a layer-stacking method. Med Phys. 33:2989–2997. DOI: 10.1118/1.2219771. PMID: 16964877.
Article20. Futami Y, Kanai T, Fujita M, Tomura H, Higashi A, Matsufuji N, et al. 1999; Broad-beam three-dimensional irradiation system for heavy-ion radiotherapy at HIMAC. Nucl Instrum Methods Phys Res Sect A. 430:143–153. DOI: 10.1016/S0168-9002(99)00194-1.
Article21. Kanematsu N, Endo M, Futami Y, Kanai T, Asakura H, Oka H, et al. 2002; Treatment planning for the layer-stacking irradiation system for three-dimensional conformal heavy-ion radiotherapy. Med Phys. 29:2823–2829. DOI: 10.1118/1.1521938. PMID: 12512716.
Article22. Eickhoff H, Bar R, Dolinskii A, Haberer T, Schlitt B, Spiller P, et al. HICAT- The German hospital-based light ion cancer therapy project. Paper presented at: Proceedings of the 2003 Particle Accelerator Conference. 2003 May 12-16; Portland, USA. p. 694–698.23. Borloni E, Rossi S. The CNAO project and the status of the construction. Paper presented at: Proceedings of NIRS-CNAO Joint Symposium on Carbon Ion Radiotherapy. 2006 Nov 27-28; Milano, Italy.24. Furukawa T, Inaniwa T, Sato S, Shirai T, Takei Y, Takeshita E, et al. 2010; Performance of the NIRS fast scanning system for heavy-ion radiotherapy. Med Phys. 37:5672–5682. DOI: 10.1118/1.3501313. PMID: 21158279.
Article25. Furukawa T, Inaniwa T, Sato S, Tomitani T, Minohara S, Noda K, et al. 2007; Design study of a raster scanning system for moving target irradiation in heavy-ion radiotherapy. Med Phys. 34:1085–1097. DOI: 10.1118/1.2558213. PMID: 17441254.
Article26. Inaniwa T, Furukawa T, Kanematsu N, Mori S, Mizushima K, Sato S, et al. 2012; Evaluation of hybrid depth scanning for carbon-ion radiotherapy. Med Phys. 39:2820–2825. DOI: 10.1118/1.4705357. PMID: 22559653.
Article27. Hara Y, Furukawa T, Mizushima K, Inaniwa T, Saotome N, Tansho R, et al. 2017; Commissioning of full energy scanning irradiation with carbon-ion beams ranging from 55.6 to 430 MeV/u at the NIRS-HIMAC. Nucl Instrum Methods Phys Res Sect B. 406:343–346. DOI: 10.1016/j.nimb.2017.02.052.
Article28. Furukawa T, Hara Y, Mizushima K, Saotome N, Tansho R, Saraya Y, et al. 2017; Development of NIRS pencil beam scanning system for carbon ion radiotherapy. Nucl Instrum Methods Phys Res Sect B. 406:361–367. DOI: 10.1016/j.nimb.2016.10.029.
Article29. Lomax A. 1999; Intensity modulation methods for proton radiotherapy. Phys Med Biol. 44:185–205. DOI: 10.1088/0031-9155/44/1/014. PMID: 10071883.
Article30. Kim J, Yoon M. 2019; Design of a compact gantry for carbon-ion beam therapy. Phys Rev Accel Beams. 22:101601. DOI: 10.1103/PhysRevAccelBeams.22.101601.
Article31. Lyman JT, Howard J. 1977; Dosimetry and instrumentation for helium and heavy ions. Int J Radiat Oncol Biol Phys. 3:81–85. DOI: 10.1016/0360-3016(77)90231-0.
Article32. Phillips TL, Fu KK, Curtis SB. 1977; Tumor biology of helium and heavy ions. Int J Radiat Oncol Biol Phys. 3:109–113. DOI: 10.1016/0360-3016(77)90236-X. PMID: 96044.
Article33. Saunders W, Castro JR, Chen GT, Collier JM, Zink SR, Pitluck S, et al. 1985; Helium-ion radiation therapy at the Lawrence Berkeley Laboratory: recent results of a Northern California Oncology Group Clinical Trial. Radiat Res Suppl. 8:S227–S234. DOI: 10.2307/3583532. PMID: 3937171.
Article34. Kempe J, Gudowska I, Brahme A. 2007; Depth absorbed dose and LET distributions of therapeutic 1H, 4He, 7Li, and 12C beams. Med Phys. 34:183–192. DOI: 10.1118/1.2400621. PMID: 17278503.35. Kantemiris I, Karaiskos P, Papagiannis P, Angelopoulos A. 2011; Dose and dose averaged LET comparison of 1H, 4He, 6Li, 8Be, 10B, 12C, 14N, and 16O ion beams forming a spread-out Bragg peak. Med Phys. 38:6585–6591. DOI: 10.1118/1.3662911. PMID: 22149840.
Article36. Tessonnier T, Marcelos T, Mairani A, Brons S, Parodi K. 2015; Phase space generation for proton and carbon ion beams for external users' applications at the Heidelberg Ion Therapy Center. Front Oncol. 5:297. DOI: 10.3389/fonc.2015.00297. PMID: 26793617.
Article37. Krämer M, Scifoni E, Schuy C, Rovituso M, Tinganelli W, Maier A, et al. 2016; Helium ions for radiotherapy? Physical and biological verifications of a novel treatment modality. Med Phys. 43:1995. DOI: 10.1118/1.4944593. PMID: 27036594.
Article38. Marafini M, Paramatti R, Pinci D, Battistoni G, Collamati F, De Lucia E, et al. 2017; Secondary radiation measurements for particle therapy applications: nuclear fragmentation produced by 4He ion beams in a PMMA target. Phys Med Biol. 62:1291–1309. DOI: 10.1088/1361-6560/aa5307. PMID: 28114124.39. Tessonnier T, Mairani A, Brons S, Sala P, Cerutti F, Ferrari A, et al. 2017; Helium ions at the Heidelberg Ion Beam Therapy Center: comparisons between FLUKA Monte Carlo code predictions and dosimetric measurements. Phys Med Biol. 62:6784–6803. DOI: 10.1088/1361-6560/aa7b12. PMID: 28762335.
Article40. Tessonnier T, Mairani A, Brons S, Haberer T, Debus J, Parodi K. 2017; Experimental dosimetric comparison of 1H, 4He, 12C and 16O scanned ion beams. Phys Med Biol. 62:3958–3982. DOI: 10.1088/1361-6560/aa6516. PMID: 28406796.41. Inaniwa T, Kanematsu N, Noda K, Kamada T. 2017; Treatment planning of intensity modulated composite particle therapy with dose and linear energy transfer optimization. Phys Med Biol. 62:5180–5197. DOI: 10.1088/1361-6560/aa68d7. PMID: 28333688.
Article42. Krämer M, Scifoni E, Schmitz F, Sokol O, Durante M. 2014; Overview of recent advances in treatment planning for ion beam radiotherapy. Eur Phys J D. 68:306. DOI: 10.1140/epjd/e2014-40843-x.
Article43. Kopp B, Mein S, Dokic I, Harrabi S, Böhlen TT, Haberer T, et al. 2020; Development and validation of single field multi-ion particle therapy treatments. Int J Radiat Oncol Biol Phys. 106:194–205. DOI: 10.1016/j.ijrobp.2019.10.008. PMID: 31610250.
Article44. Inaniwa T, Kanematsu N, Matsufuji N, Kanai T, Shirai T, Noda K, et al. 2015; Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys Med Biol. 60:3271–3286. DOI: 10.1088/0031-9155/60/8/3271. PMID: 25826534.
Article45. Inaniwa T, Suzuki M, Hyun Lee S, Mizushima K, Iwata Y, Kanematsu N, et al. 2020; Experimental validation of stochastic microdosimetric kinetic model for multi-ion therapy treatment planning with helium-, carbon-, oxygen-, and neon-ion beams. Phys Med Biol. 65:045005. DOI: 10.1088/1361-6560/ab6eba. PMID: 31968318.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Review of the Existing Relative Biological Effectiveness Models for Carbon Ion Beam Therapy
- Status and Prospects in Particle Therapy
- Technological Advances in Charged-Particle Therapy
- A review of the carbon disulfide poisoning experiences in Korean
- Current perspectives on radiotherapy in hepatocellular carcinoma management: a comprehensive review