Prog Med Phys.
2020 Mar;31(1):1-7. 10.14316/pmp.2020.31.1.1.
Review of the Existing Relative Biological Effectiveness Models for Carbon Ion Beam Therapy
- Affiliations
-
- 1Department of Nuclear and Quantum Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- 2Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2502325
- DOI: http://doi.org/10.14316/pmp.2020.31.1.1
Abstract
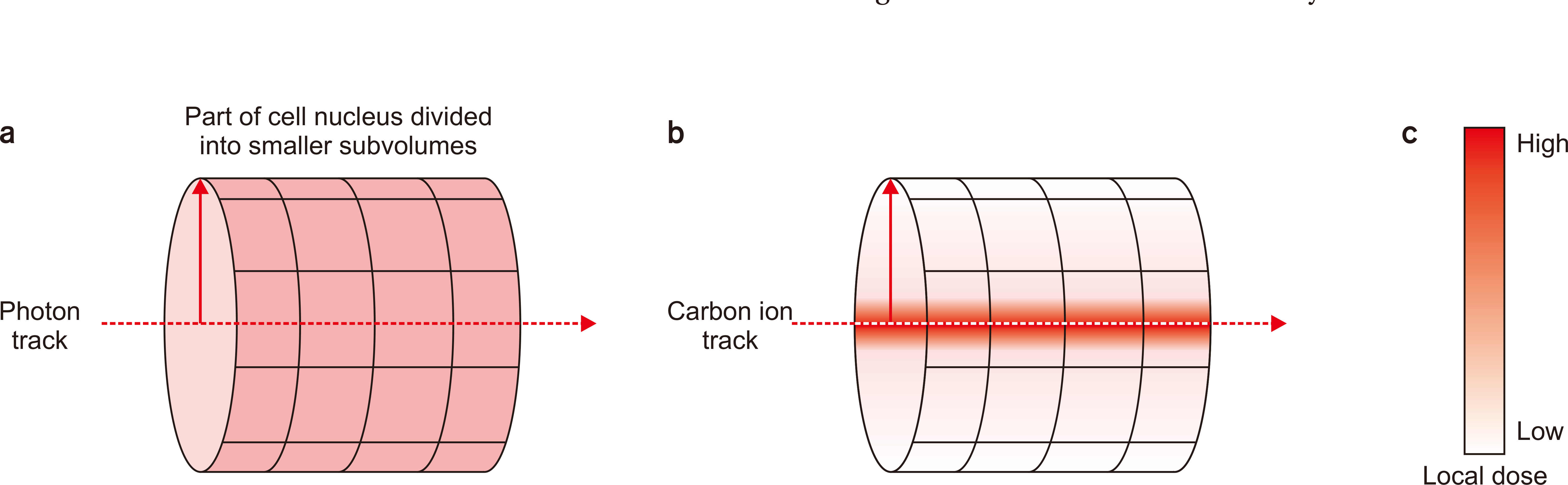
- Hadron therapy, such as carbon and helium ions, is increasingly coming to the fore for the treatment of cancers. Such hadron therapy has several advantages over conventional radiotherapy using photons and electrons physically and clinically. These advantages are due to the different physical and biological characteristics of heavy ions including high linear energy transfer and Bragg peak, which lead to the reduced exit dose, lower normal tissue complication probability and the increased relative biological effectiveness (RBE). Despite the promising prospects on the carbon ion radiation therapy, it is in dispute with which bio-mathematical models to calculate the carbon ion RBE. The two most widely used models are local effect model and microdosimetric kinetic model, which are actively utilized in Europe and Japan respectively. Such selection on the RBE model is a crucial issue in that the dose prescription for planning differs according to the models. In this study, we aim to (i) introduce the concept of RBE, (ii) clarify the determinants of RBE, and (iii) compare the existing RBE models for carbon ion therapy.
Keyword
Figure
Reference
-
1. Durante M, Debus J. 2018; Heavy charged particles: does improved precision and higher biological effectiveness translate to better outcome in patients? Semin Radiat Oncol. 28:160–167. DOI: 10.1016/j.semradonc.2017.11.004. PMID: 29735192.2. Karger CP, Peschke P. 2017; RBE and related modeling in carbon-ion therapy. Phys Med Biol. 63:01TR02. DOI: 10.1088/1361-6560/aa9102. PMID: 28976361.
Article3. Fossati P, Matsufuji N, Kamada T, Karger CP. 2018; Radiobiological issues in prospective carbon ion therapy trials. Med Phys. 45:e1096–e1110. DOI: 10.1002/mp.12506. PMID: 30421806.
Article4. Loeffler JS, Durante M. 2013; Charged particle therapy--optimization, challenges and future directions. Nat Rev Clin Oncol. 10:411–424. DOI: 10.1038/nrclinonc.2013.79. PMID: 23689752.
Article5. Wang T, Xiao P, Jia S, Yuan K, Yang H. 2014; [The basic structure of heavy-ion tumor therapy facility]. Zhongguo Yi Liao Qi Xie Za Zhi. 38:427–429, 438. Chinese. PMID: 25980131.6. Ebner DK, Kamada T. 2016; The emerging role of carbon-ion radiotherapy. Front Oncol. 6:140. DOI: 10.3389/fonc.2016.00140. PMID: 27376030. PMCID: PMC4894867.
Article7. Mohamad O, Makishima H, Kamada T. 2018; Evolution of carbon ion radiotherapy at the National Institute of Radiological Sciences in Japan. Cancers (Basel). 10:E66. DOI: 10.3390/cancers10030066. PMID: 29509684. PMCID: PMC5876641.
Article8. Lazar AA, Schulte R, Faddegon B, Blakely EA, Roach M 3rd. 2018; Clinical trials involving carbon-ion radiation therapy and the path forward. Cancer. 124:4467–4476. DOI: 10.1002/cncr.31662. PMID: 30307603. PMCID: PMC6540799.
Article9. World Health Organization. International Clinical Trials Registry Platform Search Portal. Geneva: World Health Organization. Available from: http://apps.who.int/trialsearch/default.aspx . [cited 2020 Jan 6].10. Grün R, Friedrich T, Elsässer T, Krämer M, Zink K, Karger CP, et al. 2012; Impact of enhancements in the local effect model (LEM) on the predicted RBE-weighted target dose distribution in carbon ion therapy. Phys Med Biol. 57:7261–7274. DOI: 10.1088/0031-9155/57/22/7261. PMID: 23075883.
Article11. Bentzen SM, Parliament M, Deasy JO, Dicker A, Curran WJ, Williams JP, et al. 2010; Biomarkers and surrogate endpoints for normal-tissue effects of radiation therapy: the importance of dose-volume effects. Int J Radiat Oncol Biol Phys. 76(3(Suppl)):S145–S150. DOI: 10.1016/j.ijrobp.2009.08.076. PMID: 20171510. PMCID: PMC3431963.
Article12. Lühr A, von Neubeck C, Krause M, Troost EGC. 2018; Relative biological effectiveness in proton beam therapy - current knowledge and future challenges. Clin Transl Radiat Oncol. 9:35–41. DOI: 10.1016/j.ctro.2018.01.006. PMID: 29594249. PMCID: PMC5862688.
Article13. McMahon SJ. 2018; The linear quadratic model: usage, interpretation and challenges. Phys Med Biol. 64:01TR01. DOI: 10.1088/1361-6560/aaf26a. PMID: 30523903.
Article14. Schlaff CD, Krauze A, Belard A, O’Connell JJ, Camphausen KA. 2014; Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol. 9:88. DOI: 10.1186/1748-717X-9-88. PMID: 24679134. PMCID: PMC4002206.
Article15. Grün R, Friedrich T, Traneus E, Scholz M. 2019; Is the dose-averaged LET a reliable predictor for the relative biological effectiveness? Med Phys. Phys Med Biol. 46:1064–1074. DOI: 10.1002/mp.13347. PMID: 30565705.16. Stavrev P, Stavreva N, Ruggieri R, Nahum A. 2015; On differences in radiosensitivity estimation: TCP experiments versus survival curves. a theoretical study. a theoretical study. 60:N293–N299. DOI: 10.1088/0031-9155/60/15/N293. PMID: 26215150.
Article17. Abolfath R, Peeler CR, Newpower M, Bronk L, Grosshans D, Mohan R. 2017; A model for relative biological effectiveness of therapeutic proton beams based on a global fit of cell survival data. Sci Rep. 7:8340. DOI: 10.1038/s41598-017-08622-6. PMID: 28827691. PMCID: PMC5567137.
Article18. Glatstein E. 2011; The omega on alpha and beta. Int J Radiat Oncol Biol Phys. 81:319–320. DOI: 10.1016/j.ijrobp.2011.01.011. PMID: 21871342.
Article19. Scholz M, Kellerer AM, Kraft-Weyrather W, Kraft G. 1997; Computation of cell survival in heavy ion beams for therapy. the model and its approximation. Radiat Environ Biophys. 36:59–66. DOI: 10.1007/s004110050055. PMID: 9128899.20. Elsässer T, Scholz M. 2007; Cluster effects within the local effect model. Radiat Res. 167:319–329. DOI: 10.1667/RR0467.1. PMID: 17316069.
Article21. Elsässer T, Krämer M, Scholz M. 2008; Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int J Radiat Oncol Biol Phys. 71:866–872. DOI: 10.1016/j.ijrobp.2008.02.037. PMID: 18430521.
Article22. Elsässer T, Weyrather WK, Friedrich T, Durante M, Iancu G, Krämer M, et al. 2010; Quantification of the relative biological effectiveness for ion beam radiotherapy: direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int J Radiat Oncol Biol Phys. 78:1177–1183. DOI: 10.1016/j.ijrobp.2010.05.014. PMID: 20732758.
Article23. Stewart RD, Carlson DJ, Butkus MP, Hawkins R, Friedrich T, Scholz M. 2018; A comparison of mechanism-inspired models for particle relative biological effectiveness (RBE). Med Phys. 45:e925–e952. DOI: 10.1002/mp.13207. PMID: 30421808.
Article24. Scholz M, Kraft G. 1996; Track structure and the calculation of biological effects of heavy charged particles. Adv Space Res. 18:5–14. DOI: 10.1016/0273-1177(95)00784-C. PMID: 11538986.
Article25. Elsässer T, Cunrath R, Krämer M, Scholz M. 2008; Impact of track structure calculations on biological treatment planning in ion radiotherapy. New J Phys. 10:075005. DOI: 10.1088/1367-2630/10/7/075005.
Article26. Kellerer AM, Rossi HH. 2012; A generalized formulation of dual radiation action. Radiat Res. 178:AV204–AV213. DOI: 10.1667/RRAV17.1. PMID: 22870971.
Article27. Hawkins RB. 1994; A statistical theory of cell killing by radiation of varying linear energy transfer. Radiat Res. 140:366–374. DOI: 10.2307/3579114. PMID: 7972689.
Article28. Kase Y, Kanai T, Matsumoto Y, Furusawa Y, Okamoto H, Asaba T, et al. 2006; Microdosimetric measurements and estimation of human cell survival for heavy-ion beams. Radiat Res. 166:629–638. DOI: 10.1667/RR0536.1. PMID: 17007551.
Article29. Inaniwa T, Furukawa T, Kase Y, Matsufuji N, Toshito T, Matsumoto Y, et al. 2010; Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys Med Biol. 55:6721–6737. DOI: 10.1088/0031-9155/55/22/008. PMID: 21030747.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basics of particle therapy II: relative biological effectiveness
- Status and Prospects in Particle Therapy
- Carbon Ion Therapy: A Review of an Advanced Technology
- Technological Advances in Charged-Particle Therapy
- Development of Program for Relative Biological Effectiveness (RBE) Analysis of Particle Beam Therapy