Yeungnam Univ J Med.
2020 Jul;37(3):217-225. 10.12701/yujm.2020.00248.
Mineralization-inducing potentials of calcium silicate-based pulp capping materials in human dental pulp cells
- Affiliations
-
- 1Department of Dentistry, Yeungnam University Hospital, Daegu, Korea
- KMID: 2506521
- DOI: http://doi.org/10.12701/yujm.2020.00248
Abstract
- Background
To provide a long-term bacterial seal through the formation of reparative dentin bridge, calcium silicate-based pulp capping materials have been used at sites of pulpal exposure. The aim of this study was to evaluate the mineralization-inducing potentials of calcium silicate-based pulp capping materials (ProRoot MTA [PR], Biodentine [BD], and TheraCal LC [TC]) in human dental pulp cells (HDPCs).
Methods
Specimens of test materials were placed in deionized water for various incubation times to measure the pH variation and the concentration of calcium released. The morphology of HDPCs cultured on the specimens was examined using a confocal laser scanning microscope (CLSM). Alizarin red S staining and alkaline phosphatase assays were used to evaluate mineralization-inducing potentials of the capping materials.
Results
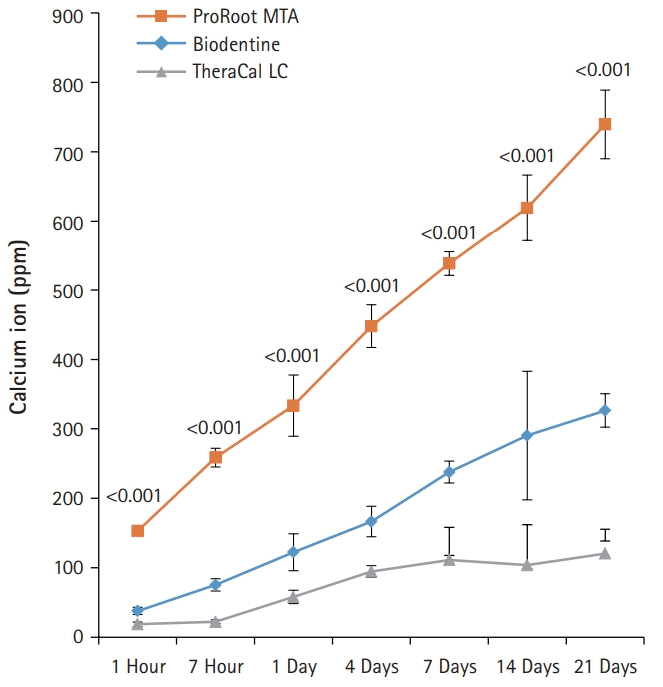
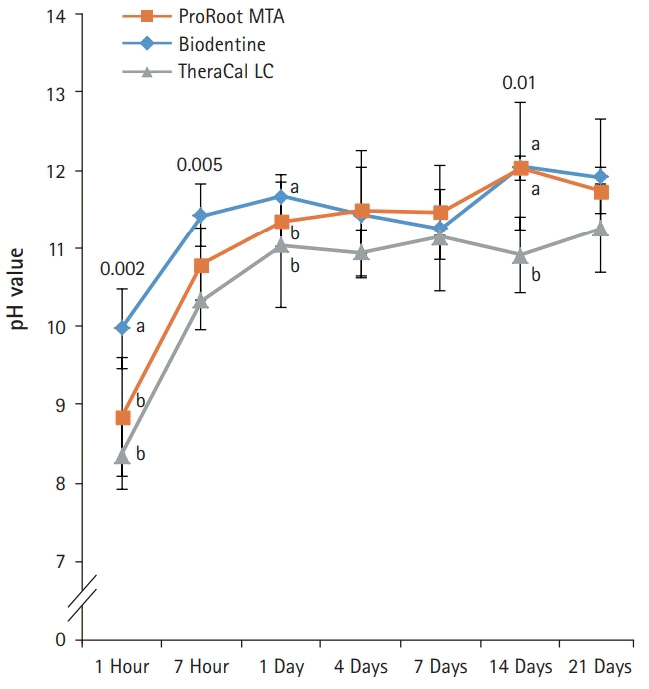
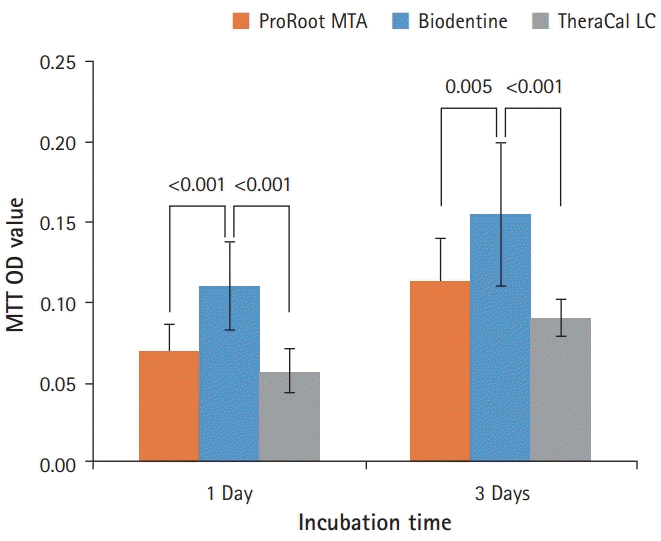
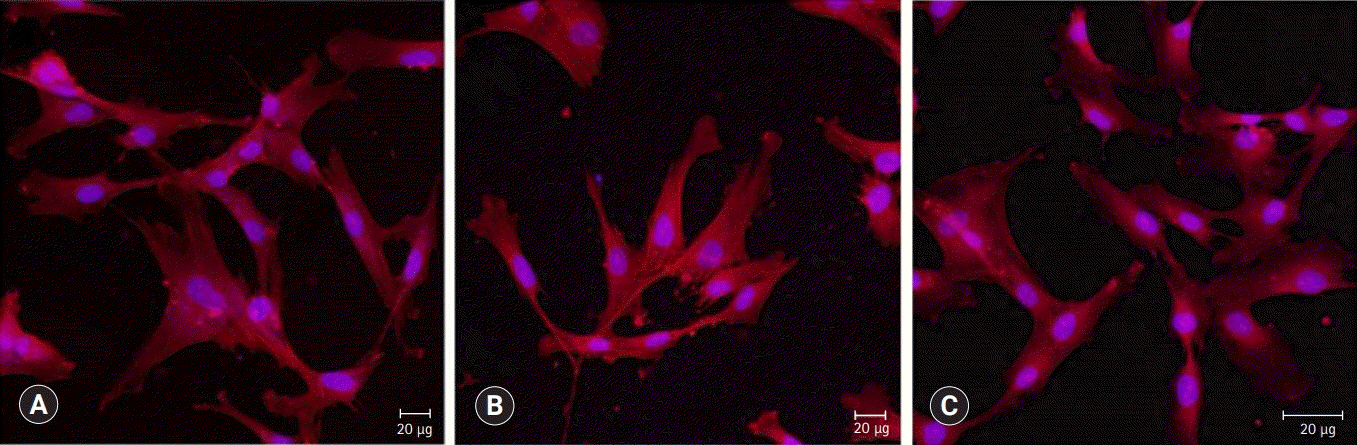
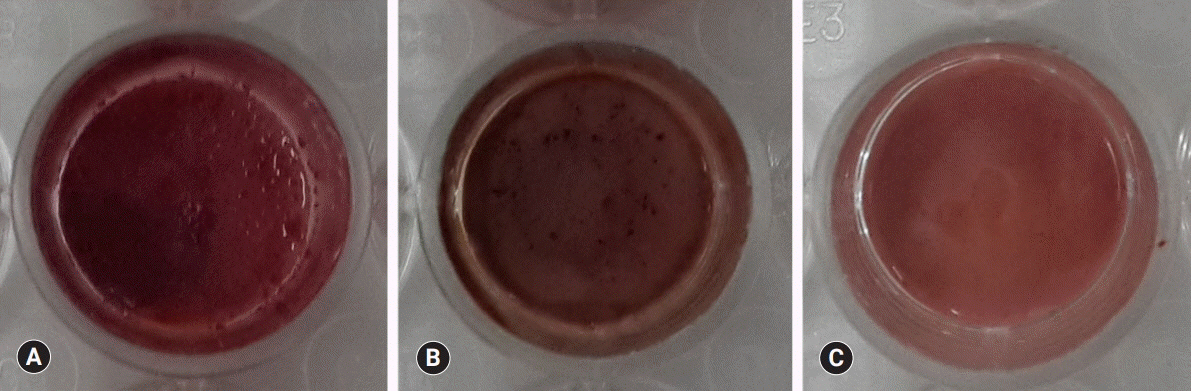
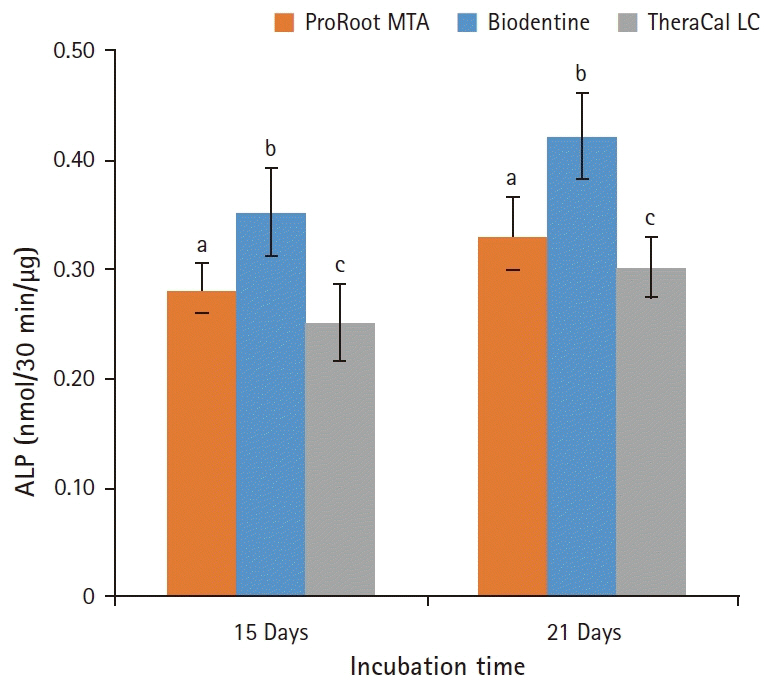
BD showed the highest calcium release in all test periods, followed by PR and TC. (p<0.05). All experimental groups showed high alkalinity after 1 day, except at 14 days. BD showed the highest cell viability compared with PR and TC after 1 and 3 days, while TC showed the lowest value (p<0.05). The CLSM analysis showed that cells were well adhered and expressed actin filaments for all pulp capping materials. Mineralization by PR and BD groups was higher than that by TC group based on alizarin red S staining. BD showed significantly higher alkaline phosphatase activity than PR and TC, while TC showed the lowest value (p<0.05).
Conclusion
Within the limitations of the in vitro study, BD had higher mineralization-inducing potential than PR and TC.
Figure
Cited by 1 articles
-
Cytotoxicity of dental self-curing resin for a temporary crown: an
in vitro study
Jae-wan Ko, Joon Sakong, Sohee Kang
J Yeungnam Med Sci. 2023;40(Suppl):S1-S8. doi: 10.12701/jyms.2023.00080.
Reference
-
References
1. Smith AJ. Formation and repair of dentin in the adult. In : Hargreaves KM, Goodis HE, Tay FR, editors. Seltzer and Bender's dental pulp. 2nd ed. Hanover Park (IL): Quintessence Publishing;2012. p. 27–46.2. Sangwan P, Sangwan A, Duhan J, Rohilla A. Tertiary dentinogenesis with calcium hydroxide: a review of proposed mechanisms. Int Endod J. 2013; 46:3–19.
Article3. Okiji T, Yoshiba K. Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int J Dent. 2009; 2009:464280.
Article4. Mente J, Geletneky B, Ohle M, Koch MJ, Friedrich Ding PG, Wolff D, et al. Mineral trioxide aggregate or calcium hydroxide direct pulp capping: an analysis of the clinical treatment outcome. J Endod. 2010; 36:806–13.
Article5. Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc. 2008; 139:305–15.6. Chang SW, Lee SY, Ann HJ, Kum KY, Kim EC. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J Endod. 2014; 40:1194–200.
Article7. Gandolfi MG, Siboni F, Polimeni A, Bossu M, Riccitiello F, Rengo S, et al. In vitro screening of the apatite-forming ability, biointeractivity and physical properties of a tricalcium silicate material for endodontics and restorative dentistry. Dent J. 2013; 1:41–60.
Article8. Suh B, Cannon M, Yin R, Martin DE. Polymerizable dental pulp healing, capping, and lining material and method for use. International patent A61K33/42. 2008; Aug. 28.9. Hebling J, Lessa FC, Nogueira I, Carvalho RM, Costa CA. Cytotoxicity of resin-based light-cured liners. Am J Dent. 2009; 22:137–42.10. Camilleri J, Laurent P, About I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod. 2014; 40:1846–54.11. Rashid F, Shiba H, Mizuno N, Mouri Y, Fujita T, Shinohara H, et al. The effect of extracellular calcium ion on gene expression of bone-related proteins in human pulp cells. J Endod. 2003; 29:104–7.
Article12. Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, et al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006; 39:415–22.
Article13. Estrela C, Holland R. Calcium hydroxide: study based on scientific evidences. J Appl Oral Sci. 2003; 11:269–82.
Article14. Formosa LM, Mallia B, Camilleri J. The chemical properties of light- and chemical-curing composites with mineral trioxide aggregate filler. Dent Mater. 2013; 29:e11–9.
Article15. Grech L, Mallia B, Camilleri J. Characterization of set Intermediate Restorative Material, Biodentine, Bioaggregate and a prototype calcium silicate cement for use as root-end filling materials. Int Endod J. 2013; 46:632–41.
Article16. Moghaddame-Jafari S, Mantellini MG, Botero TM, McDonald NJ, Nor JE. Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod. 2005; 31:387–91.
Article17. Maeda H, Nakano T, Tomokiyo A, Fujii S, Wada N, Monnouchi S, et al. Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J Endod. 2010; 36:647–52.
Article18. Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008; 41:408–17.
Article19. Camilleri J. Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent Mater. 2014; 30:709–15.
Article20. Poggio C, Arciola CR, Beltrami R, Monaco A, Dagna A, Lombardini M, et al. Cytocompatibility and antibacterial properties of capping materials. ScientificWorldJournal. 2014; 2014:181945.
Article21. Corral Nunez CM, Bosomworth HJ, Field C, Whitworth JM, Valentine RA. Biodentine and mineral trioxide aggregate induce similar cellular responses in a fibroblast cell line. J Endod. 2014; 40:406–11.
Article22. Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996; 12:463–518.
Article23. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000; 97:13625–30.24. Laurent P, Camps J, About I. Biodentine(TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012; 45:439–48.
Article25. Nowicka A, Wilk G, Lipski M, Kolecki J, Buczkowska-Radlinska J. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca(OH)2, MTA, Biodentine, and dentin bonding system in human teeth. J Endod. 2015; 41:1234–40.
Article26. Tziafa C, Koliniotou-Koumpia E, Papadimitriou S, Tziafas D. Dentinogenic responses after direct pulp capping of miniature swine teeth with Biodentine. J Endod. 2014; 40:1967–71.27. Chung H, Kim M, Ko H, Yang W. Evaluation of physical and biologic properties of the mixture of mineral trioxide aggregate and 4-META/MMA-TBB resin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:e6–11.
Article28. Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991; 70:1450–5.
Article29. Michelsen VB, Lygre H, Skalevik R, Tveit AB, Solheim E. Identification of organic eluates from four polymer-based dental filling materials. Eur J Oral Sci. 2003; 111:263–71.
Article30. Kwon JH, Park HC, Zhu T, Yang HC. Inhibition of odontogenic differentiation of human dental pulp cells by dental resin monomers. Biomater Res. 2015; 19:8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Color Change in Tooth Induced by Various Calcium Silicate-Based Pulp-Capping Materials
- Comparison of the Microleakage and Shear Bond Strength to Dentine of Different Tricalcium Silicate-based Pulp Capping Materials
- Effects of the exposure site on histological pulpal responses after direct capping with 2 calcium-silicate based cements in a rat model
- Considerations during crown reattachment procedure over the pulpal exposure: case report
- A bioactivity study of Portland cement mixed with beta-glycerophosphosphate on human pulp cell