Cancer Res Treat.
2020 Jul;52(3):798-814. 10.4143/crt.2019.498.
Long Non-coding RNA CCAT1 Sponges miR-454 to Promote Chemoresistance of Ovarian Cancer Cells to Cisplatin by Regulation of Surviving
- Affiliations
-
- 1Department of Gynaecology, The 2nd Affiliated Hospital of Harbin Medical University, Harbin, China
- KMID: 2504461
- DOI: http://doi.org/10.4143/crt.2019.498
Abstract
- Purpose
Colon cancer-associated transcript 1 (CCAT1) was identified as an oncogenic long non-coding RNA (lncRNA) in a variety of cancers. However, there was a lack of understanding of the mechanism by which CCAT1 conferred cisplatin (also known as DDP) resistance in ovarian cancer cells.
Materials and Methods
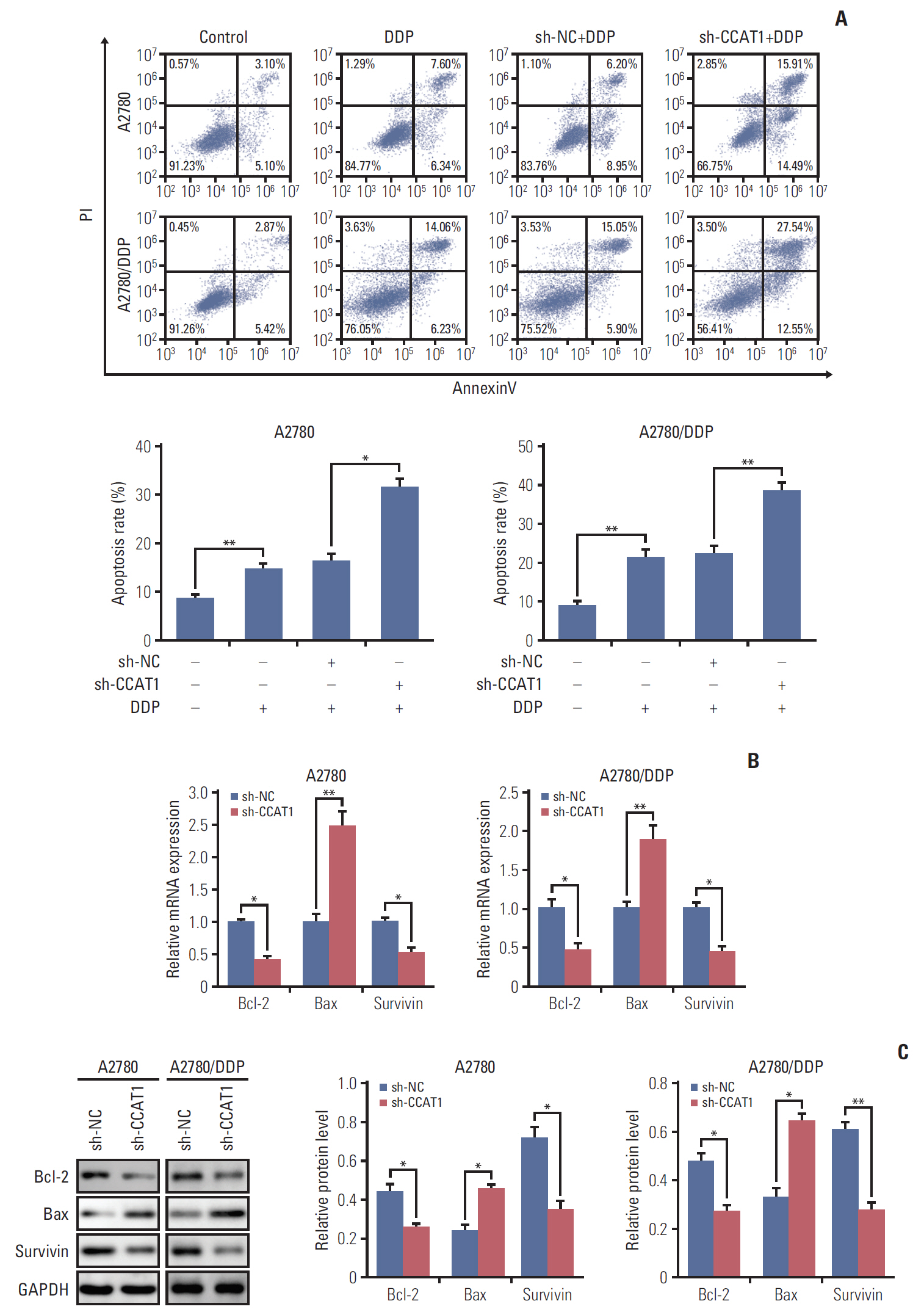
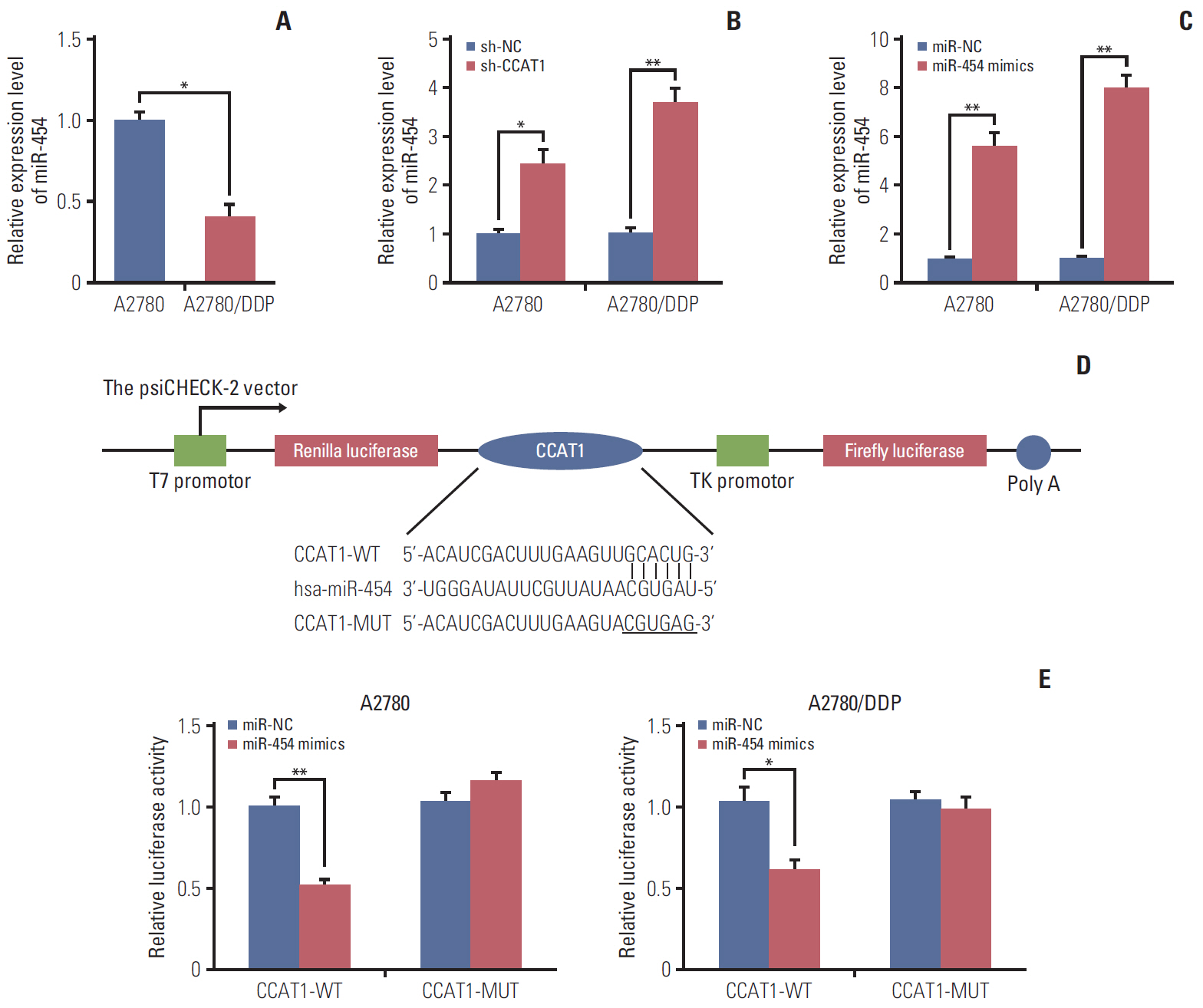
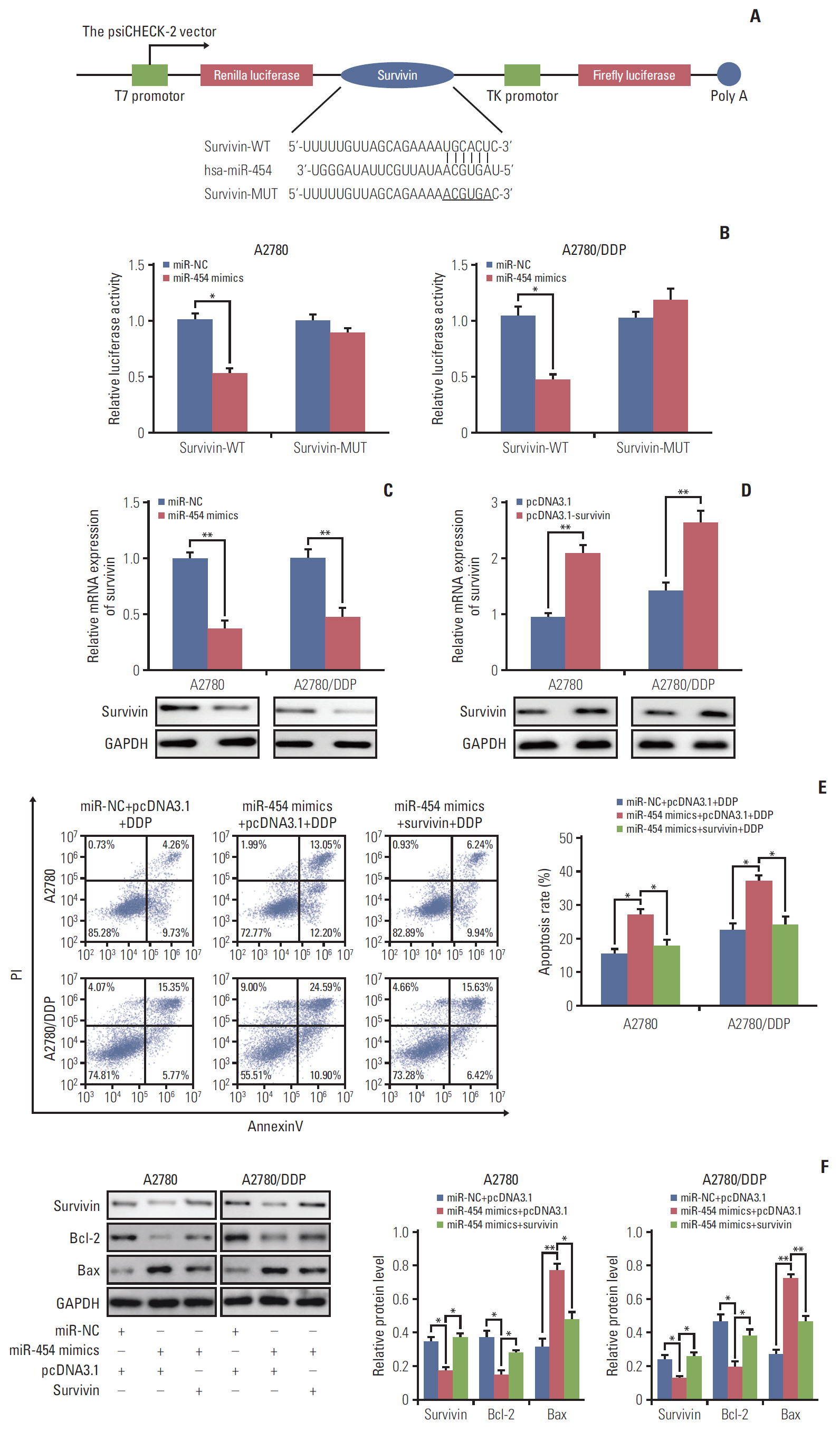
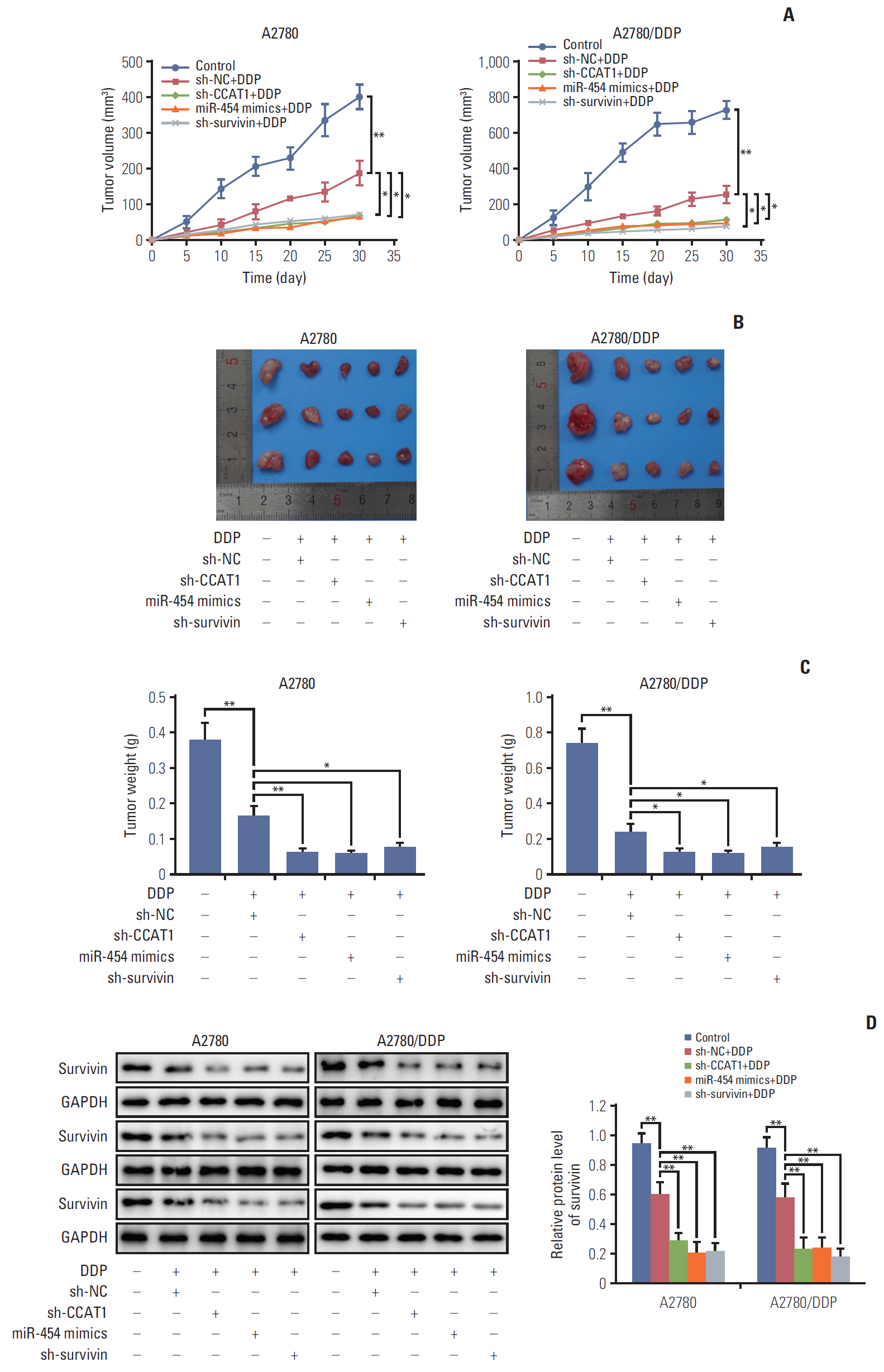
Cell viability of A2780, SKOV3, A2780/DDP, and SKOV3/DDP cells upon cisplatin treatment was monitored by MTT assay. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) detected the expression levels of CCAT1 and miR-454. The effect of sh-CCAT1 on cisplatin response was investigated in xenografts study. Bioinformatic analysis, luciferase reporter assay and qRT-PCR were conducted to validate the direct interaction among CCAT1, miR-454, and survivin. Apoptosis was determined by flow cytometry after dual staining of Annexin-V-FITC/propidium iodide, and the expression of apoptosis-related proteins Bcl-2, Bax and survivin were detected by qRT-PCR and Western blotting. Xenograft study was conducted to monitor in vivo tumor formation.
Results
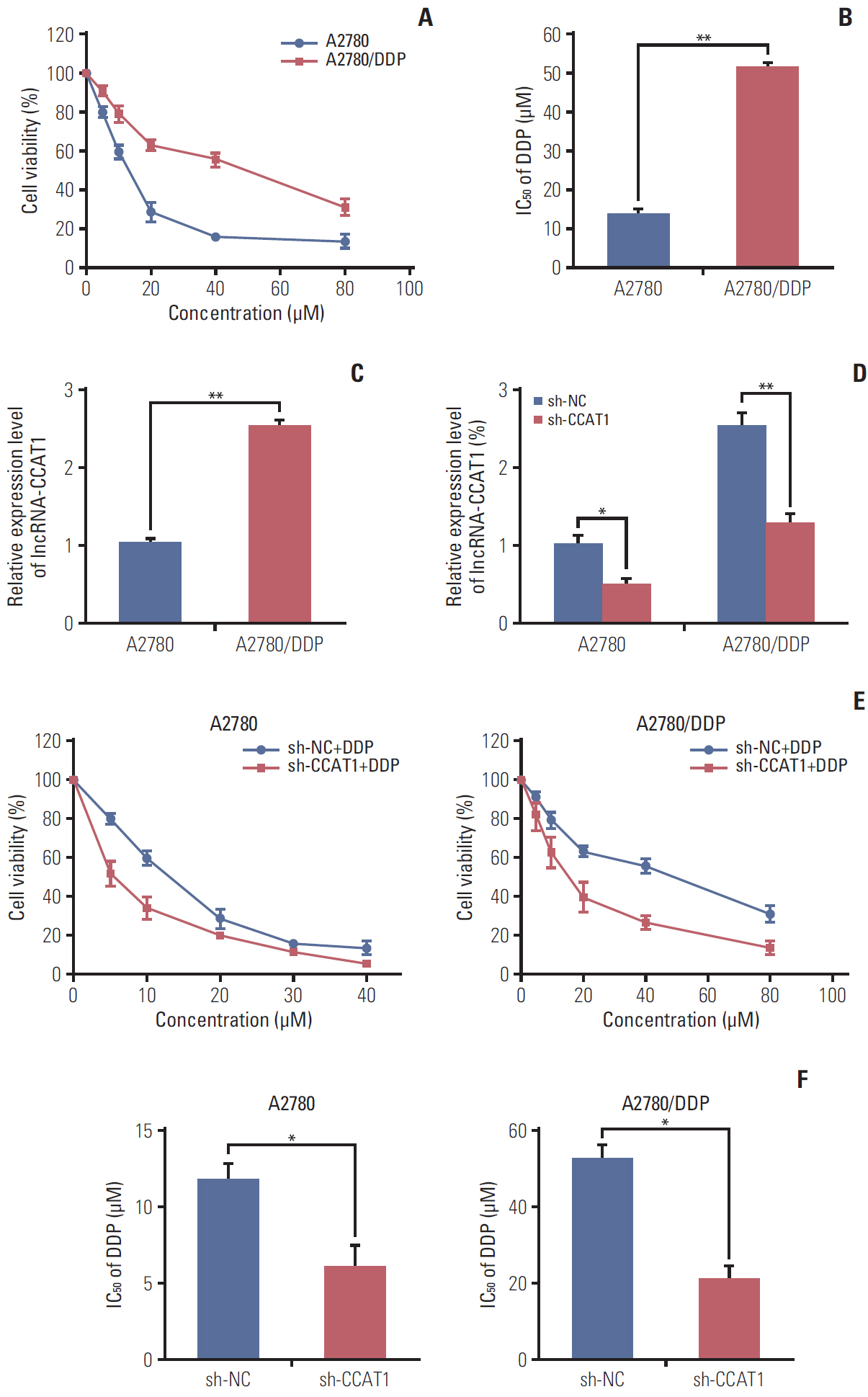
CCAT1 was highly expressed in cisplatin-resistant ovarian cancer cell line A2780/DDP and SKOV3/DDP. Knockdown of CCAT1 restored sensitivity to cisplatin in vitro and in vivo. Our data revealed that silencing of CCAT1 promoted cisplatin-induced apoptosis via modulating the expression of pro- or anti-apoptotic proteins Bax, Bcl-2, and survivin. CCAT1 directly interacted with miR-454, and miR-454 overexpression potentiated cisplatin-induced apoptosis. Survivin was identified as a functional target of miR-454, restoration of survivin attenuated the effect of miR-454 on cisplatin response. In addition, miR-454 inhibitor or overexpression of survivin was found to abolish sh-CCAT1–induced apoptosis upon cisplatin treatment.
Conclusion
CCAT1/miR-454/survivin axis conferred cisplatin resistance in ovarian cancer cells.
Keyword
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30.
Article2. Jiang X, Tang H, Chen T. Epidemiology of gynecologic cancers in China. J Gynecol Oncol. 2018; 29:e7.
Article3. Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 2017; 34:103.
Article4. Nikpayam E, Tasharrofi B, Sarrafzadeh S, Ghafouri-Fard S. The role of long non-coding RNAs in ovarian cancer. Iran Biomed J. 2017; 21:3–15.
Article5. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363:943–53.
Article6. Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014; 15:7–21.
Article7. Chen J, Zhang K, Song H, Wang R, Chu X, Chen L. Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells. Oncotarget. 2016; 7:62474–89.
Article8. Nissan A, Stojadinovic A, Mitrani-Rosenbaum S, Halle D, Grinbaum R, Roistacher M, et al. Colon cancer associated transcript-1: a novel RNA expressed in malignant and pre-malignant human tissues. Int J Cancer. 2012; 130:1598–606.
Article9. Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015; 34:18.
Article10. Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, et al. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015; 6:e1583.
Article11. Liu SP, Yang JX, Cao DY, Shen K. Identification of differentially expressed long non-coding RNAs in human ovarian cancer cells with different metastatic potentials. Cancer Biol Med. 2013; 10:138–41.12. Wang Q, Zhang W, Hao S. LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle. 2017; 16:795–801.
Article13. Yang X, Zheng F, Xing H, Gao Q, Wei W, Lu Y, et al. Resistance to chemotherapy-induced apoptosis via decreased caspase-3 activity and overexpression of antiapoptotic proteins in ovarian cancer. J Cancer Res Clin Oncol. 2004; 130:423–8.
Article14. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011; 146:353–8.
Article15. Wang S, Huang X, Lee CK, Liu B. Elevated expression of erbB3 confers paclitaxel resistance in erbB2-overexpressing breast cancer cells via upregulation of Survivin. Oncogene. 2010; 29:4225–36.
Article16. Zhong Y, Gao D, He S, Shuai C, Peng S. Dysregulated expression of long noncoding RNAs in ovarian cancer. Int J Gynecol Cancer. 2016; 26:1564–70.
Article17. Lai XJ, Cheng HF. LncRNA colon cancer-associated transcript 1 (CCAT1) promotes proliferation and metastasis of ovarian cancer via miR-1290. Eur Rev Med Pharmacol Sci. 2018; 22:322–8.18. Inoue S, Salah-Eldin AE, Omoteyama K. Apoptosis and anticancer drug resistance. Hum Cell. 2001; 14:211–21.19. Helm CW, States JC. Enhancing the efficacy of cisplatin in ovarian cancer treatment - could arsenic have a role. J Ovarian Res. 2009; 2:2.
Article20. Fraser M, Leung B, Jahani-Asl A, Yan X, Thompson WE, Tsang BK. Chemoresistance in human ovarian cancer: the role of apoptotic regulators. Reprod Biol Endocrinol. 2003; 1:66.21. Hu B, Zhang H, Wang Z, Zhang F, Wei H, Li L. LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol Ther. 2017; 18:974–83.
Article22. Liang HL, Hu AP, Li SL, Xie JP, Ma QZ, Liu JY. MiR-454 prompts cell proliferation of human colorectal cancer cells by repressing CYLD expression. Asian Pac J Cancer Prev. 2015; 16:2397–402.
Article23. Zhou L, Qu YM, Zhao XM, Yue ZD. Involvement of miR-454 overexpression in the poor prognosis of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2016; 20:825–9.24. Li Q, Liu J, Meng X, Pang R, Li J. MicroRNA-454 may function as an oncogene via targeting AKT in triple negative breast cancer. J Biol Res (Thessalon). 2017; 24:10.
Article25. Wang X, Liu B, Wen F, Song Y. MicroRNA-454 inhibits the malignant biological behaviours of gastric cancer cells by directly targeting mitogen-activated protein kinase 1. Oncol Rep. 2018; 39:1494–504.
Article26. Zhao X, Li X, Zhou L, Ni J, Yan W, Ma R, et al. LncRNA HOXA11-AS drives cisplatin resistance of human LUAD cells via modulating miR-454-3p/Stat3. Cancer Sci. 2018; 109:3068–79.
Article27. Chen X, Duan N, Zhang C, Zhang W. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J Cancer. 2016; 7:314–23.
Article28. Xu Y, Wang S, Chan HF, Lu H, Lin Z, He C, et al. Dihydromyricetin induces apoptosis and reverses drug resistance in ovarian cancer cells by p53-mediated downregulation of survivin. Sci Rep. 2017; 7:46060.
Article29. Chen Q, Zhang H. Smac combined with DDP can inhibit drug resistance of ovarian cancer through regulation of Survivin expression. Cancer Biomark. 2018; 22:1–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-338-3p Enhances Ovarian Cancer Cell Sensitivity to Cisplatin by Downregulating WNT2B
- Upregulation of long non-coding RNA XIST has anticancer effects on epithelial ovarian cancer cells through inverse downregulation of hsa-miR-214-3p
- Long Non-Coding RNA LINC00525 Promotes the Stemness and Chemoresistance of Colorectal Cancer by Targeting miR-507/ELK3 Axis
- MEG3 LncRNA from Exosomes Released from Cancer-Associated Fibroblasts Enhances Cisplatin Chemoresistance in SCLC via a MiR-15a-5p/CCNE1 Axis
- Cisplatin-induced PANDAR-Chemo-EVs contribute to a more aggressive and chemoresistant ovarian cancer phenotype through the SRSF9-SIRT4/ SIRT6 axis