Cancer Res Treat.
2020 Jul;52(3):789-797. 10.4143/crt.2019.749.
Soluble Axl Is a Novel Diagnostic Biomarker of Hepatocellular Carcinoma in Chinese Patients with Chronic Hepatitis B Virus Infection
- Affiliations
-
- 1Guangxi Medical University Cancer Hospital, Nanning, China
- 2People’s Hospital of Wudi County, Binzhou, China
- 3The Affiliated Yantai Yuhuangding Hospital of Qingdao University Institution, Yantai, China
- KMID: 2504460
- DOI: http://doi.org/10.4143/crt.2019.749
Abstract
- Purpose
The purpose of this study was to evaluate the diagnostic value of soluble Axl (sAxl) in hepatocellular carcinoma (HCC) in comparison with serum α-fetoprotein (AFP).
Materials and Methods
Eighty HCC patients, 80 liver cirrhosis patients (LC), 80 patients with hepatitis B virus (HBV) infection, and 80 healthy controls (HC) were enrolled. sAxl levels were measured by an enzyme-linked immunosorbent assay, serum AFP levelswere measured by an electrochemiluminescence immunoassay. Receiver operating characteristic (ROC) curves were used to evaluate diagnostic performances.
Results
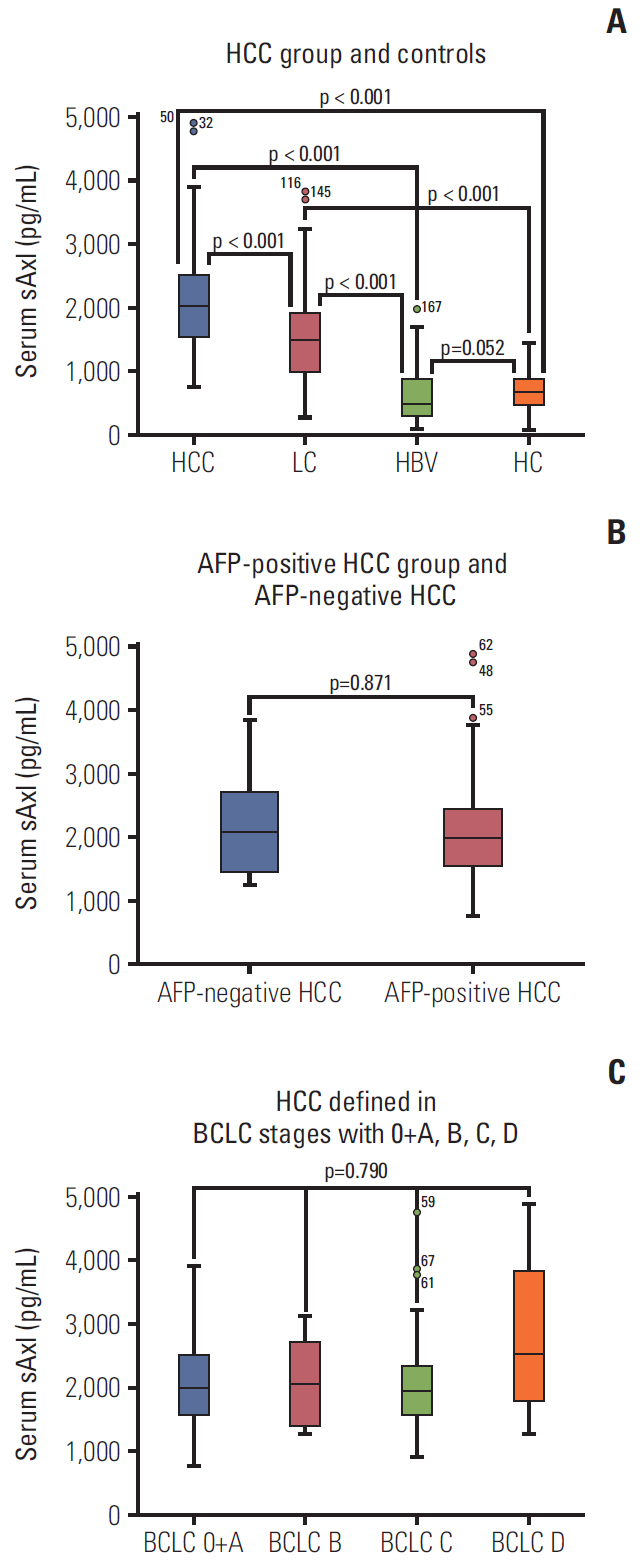
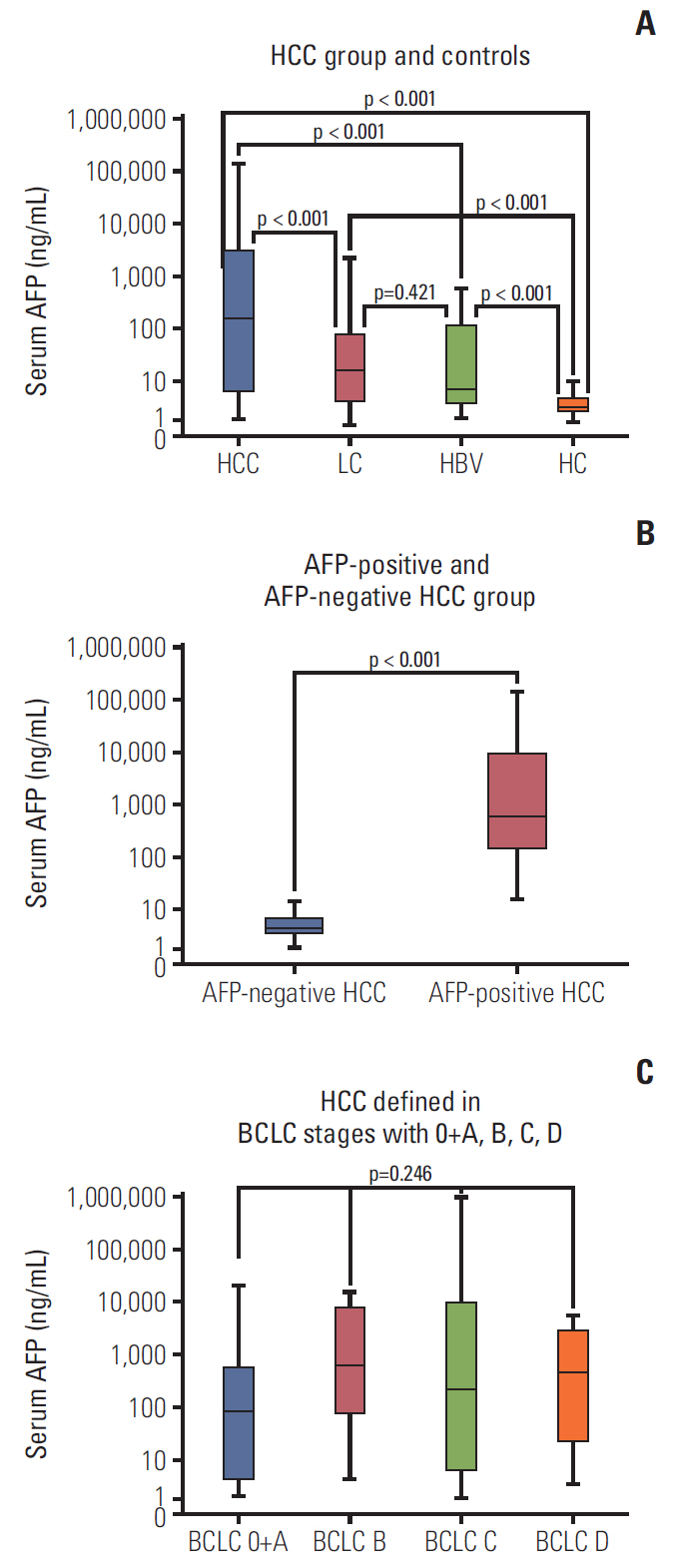
The results show that levels of sAxl were high expression in patients with HCC (p < 0.05), varied with disease state as follows: HCC > LC > HC > HBV. Logistic regression and ROC curve analysis identified the optimal cut-off for sAxl in differentiating all HCC and non-HCC patients was 1,202 pg/mL (area under the receiver operating characteristic [AUC], 0.888; 95% confidence interval [CI], 0.852 to 0.924) with sensitivity 95.0%, specificity 73.3%. Furthermore, differential diagnosis of early HCC with non-HCC patients for sAxl showed the optimal cut-off was 1,202 pg/mL (AUC, 0.881; 95% CI, 0.831 to 0.931; sensitivity, 94.1%; specificity, 73.3%). Among AFP-negative HCC patients with non-HCC patients, the cut-off was 1,301 pg/mL (AUC, 0.898; 95% CI, 0.854 to 0.942) with a sensitivity of 84.6%, a specificity of 76.3%. The optimal cut-off for sAxl in differentiating all HCC and chronic liver disease patients was 1,243 pg/mL (AUC, 0.840; 95% CI, 0.791 to 0.888) with sensitivity 93.8%, specificity 61.9%. The combination of AFP and sAxl increased diagnostic value for HCC.
Conclusion
sAxl outperforms AFP in detecting HCC, especially in early HCC and in AFP-negative HCC. Combination sAxl with AFP improved the specificity for early HCC diagnosis. In summary, sAxl is a candidate serum marker for diagnosing HCC.
Keyword
Figure
Reference
-
References
1. Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019; 41:19–28.2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article3. Abraham JA, Golubnitschaja O. Time for paradigm change in management of hepatocellular carcinoma: is a personalized approach on the horizon? Per Med. 2016; 13:455–67.
Article4. Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015; 21:10573–83.
Article5. Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004; 127(5 Suppl 1):S108–12.6. Chen H, Chen S, Li S, Chen Z, Zhu X, Dai M, et al. Combining des-gamma-carboxyprothrombin and alpha-fetoprotein for hepatocellular carcinoma diagnosing: an update meta-analysis and validation study. Oncotarget. 2017; 8:90390–401.
Article7. Zhao Y, Gao Q, Pei L, Wang C, Jin L, Liao F. Current status and future prospects of biomarkers in the diagnosis of hepatocellular carcinoma. Int J Biol Markers. 2017; 32:e361–9.
Article8. Kim SU, Park JH, Kim HS, Lee JM, Lee HG, Kim H, et al. Serum Dickkopf-1 as a biomarker for the diagnosis of hepatocellular carcinoma. Yonsei Med J. 2015; 56:1296–306.
Article9. Lim TS, Kim DY, Han KH, Kim HS, Shin SH, Jung KS, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol. 2016; 51:344–53.
Article10. Han J, Tian R, Yong B, Luo C, Tan P, Shen J, et al. Gas6/Axl mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun. 2013; 435:493–500.
Article11. Wu X, Ma W, Zhou Q, Yan H, Lim ZF, Huang M, et al. AXLGAS6 expression can predict for adverse prognosis in non-small cell lung cancer with brain metastases. J Cancer Res Clin Oncol. 2017; 143:1947–57.
Article12. Reichl P, Dengler M, van Zijl F, Huber H, Fuhrlinger G, Reichel C, et al. Axl activates autocrine transforming growth factor-beta signaling in hepatocellular carcinoma. Hepatology. 2015; 61:930–41.13. Xu J, Jia L, Ma H, Li Y, Ma Z, Zhao Y. Axl gene knockdown inhibits the metastasis properties of hepatocellular carcinoma via PI3K/Akt-PAK1 signal pathway. Tumour Biol. 2014; 35:3809–17.
Article14. Liu J, Wang K, Yan Z, Xia Y, Li J, Shi L, et al. Axl expression stratifies patients with poor prognosis after hepatectomy for hepatocellular carcinoma. PLoS One. 2016; 11:e0154767.
Article15. Ekman C, Stenhoff J, Dahlback B. Gas6 is complexed to the soluble tyrosine kinase receptor Axl in human blood. J Thromb Haemost. 2010; 8:838–44.
Article16. Dengler M, Huber H, Muller CJ, Zellmer A, Rauch P, Mikulits W. Accurate determination of soluble Axl by enzyme-linked immunosorbent assay. Assay Drug Dev Technol. 2016; 14:543–50.
Article17. Reichl P, Fang M, Starlinger P, Staufer K, Nenutil R, Muller P, et al. Multicenter analysis of soluble Axl reveals diagnostic value for very early stage hepatocellular carcinoma. Int J Cancer. 2015; 137:385–94.
Article18. Dengler M, Staufer K, Huber H, Stauber R, Bantel H, Weiss KH, et al. Soluble Axl is an accurate biomarker of cirrhosis and hepatocellular carcinoma development: results from a large scale multicenter analysis. Oncotarget. 2017; 8:46234–48.
Article19. Reichl P, Mikulits W. Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma: an update for clinicians (review). Oncol Rep. 2016; 36:613–25.
Article20. Guo J, Pan LH, Li YX, Yang XD, Li LQ, Zhang CY, et al. Efficacy of triclosan-coated sutures for reducing risk of surgical site infection in adults: a meta-analysis of randomized clinical trials. J Surg Res. 2016; 201:105–17.
Article21. Wang YT, Chen TY, Zhu J, Jiao YC, Qu CF. Primary prevention by hepatitis B vaccine on liver cancer in high incidence area of China. Zhonghua Yu Fang Yi Xue Za Zhi. 2018; 52:402–8.22. Kudo M. Evidence and consensus on the management of hepatocellular carcinoma: update 2015. Oncology. 2015; 89 Suppl 2:1–3.
Article23. Weinmann A, Koch S, Sprinzl M, Kloeckner R, Schulze-Bergkamen H, Duber C, et al. Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int. 2015; 35:591–600.
Article24. Li J, Cheng ZJ, Liu Y, Yan ZL, Wang K, Wu D, et al. Serum thioredoxin is a diagnostic marker for hepatocellular carcinoma. Oncotarget. 2015; 6:9551–63.
Article25. Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012; 13:817–26.
Article26. Staufer K, Dengler M, Huber H, Marculescu R, Stauber R, Lackner C, et al. The non-invasive serum biomarker soluble Axl accurately detects advanced liver fibrosis and cirrhosis. Cell Death Dis. 2017; 8:e3135.
Article27. Smirne C, Rigamonti C, De Benedittis C, Sainaghi PP, Bellan M, Burlone ME, et al. Gas6/TAM signaling components as novel biomarkers of liver fibrosis. Dis Markers. 2019; 2019:2304931.
Article28. Tutusaus A, de Gregorio E, Cucarull B, Cristobal H, Areste C, Graupera I, et al. A functional role of GAS6/TAM in nonalcoholic steatohepatitis progression implicates AXL as therapeutic target. Cell Mol Gastroenterol Hepatol. 2019; 9:349–68.
Article29. Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011; 30:1229–40.
Article30. Paccez JD, Vogelsang M, Parker MI, Zerbini LF. The receptor tyrosine kinase Axl in cancer: biological functions and therapeutic implications. Int J Cancer. 2014; 134:1024–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Viral Hepatitis and Vaccination
- A case of primary hepatocellular carcinoma following vertical transmission of hepatitis B virus in a child
- The management of chronic hepatitis C
- Comparative study of serum soluble interleukin-2 receptor and hepatitis C virus RNA in patients with chronic hepatitis C virus infection
- Viral Hepatitis: Focus on Clinical Manifestations of Hepatitis A, B and C