Int J Stem Cells.
2020 Jul;13(2):221-236. 10.15283/ijsc19110.
Intratracheal Transplantation of Amnion-Derived Mesenchymal Stem Cells Ameliorates Hyperoxia-Induced Neonatal Hyperoxic Lung Injury via Aminoacyl-Peptide Hydrolase
- Affiliations
-
- 1Department of Pediatrics, Qilu Hospital of Shandong University, Ji’nan, China
- 2Department of Pediatrics, Yidu Central Hospital of Weifang, Qingzhou, China
- 3Department of Pediatrics, Qingdao Women and Children’s Hospital, Qingdao, China
- 4Stem Cell and Regenerative Medicine Research Center of Shandong University, Ji’nan, China
- KMID: 2504335
- DOI: http://doi.org/10.15283/ijsc19110
Abstract
- Background and Objectives
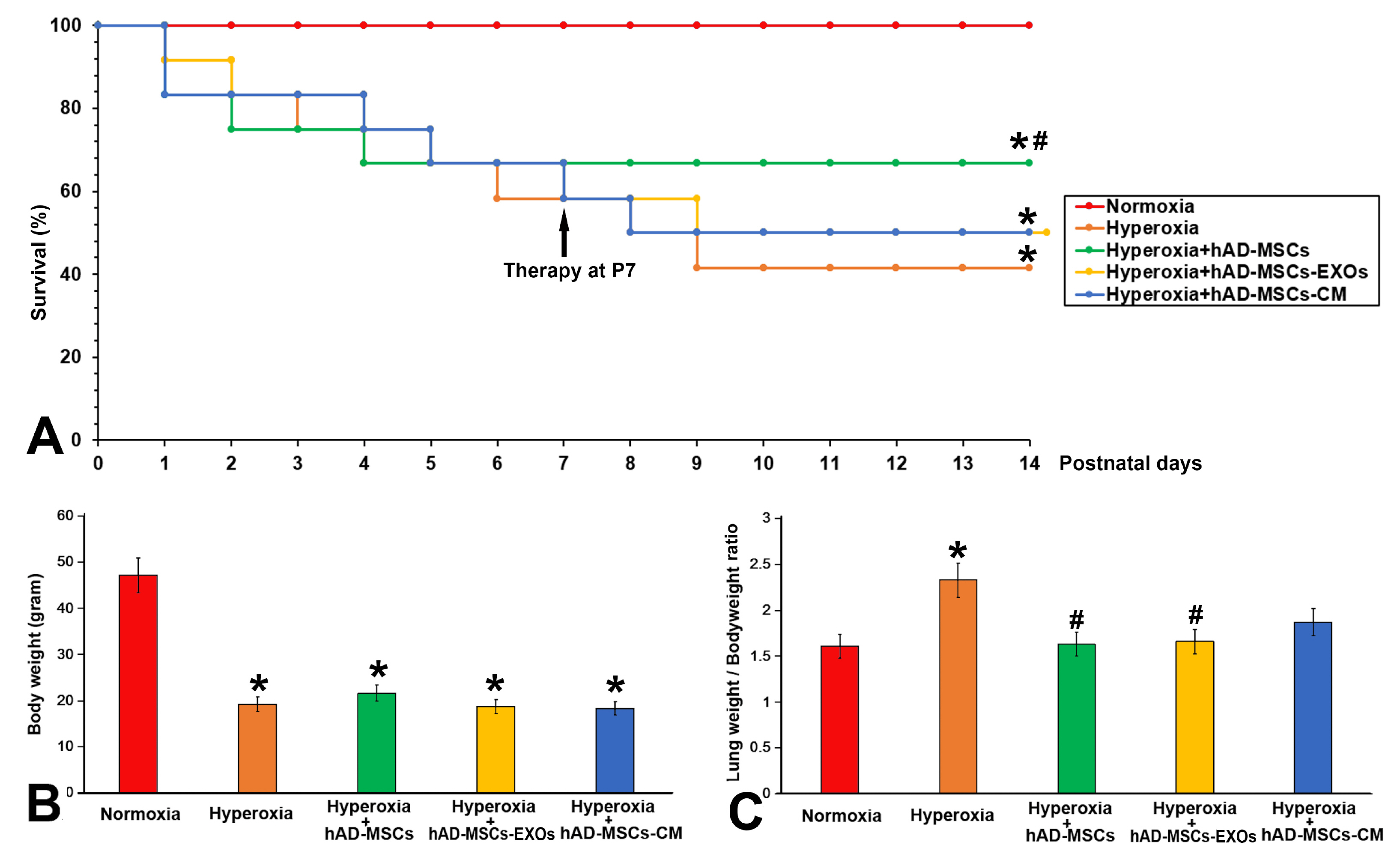
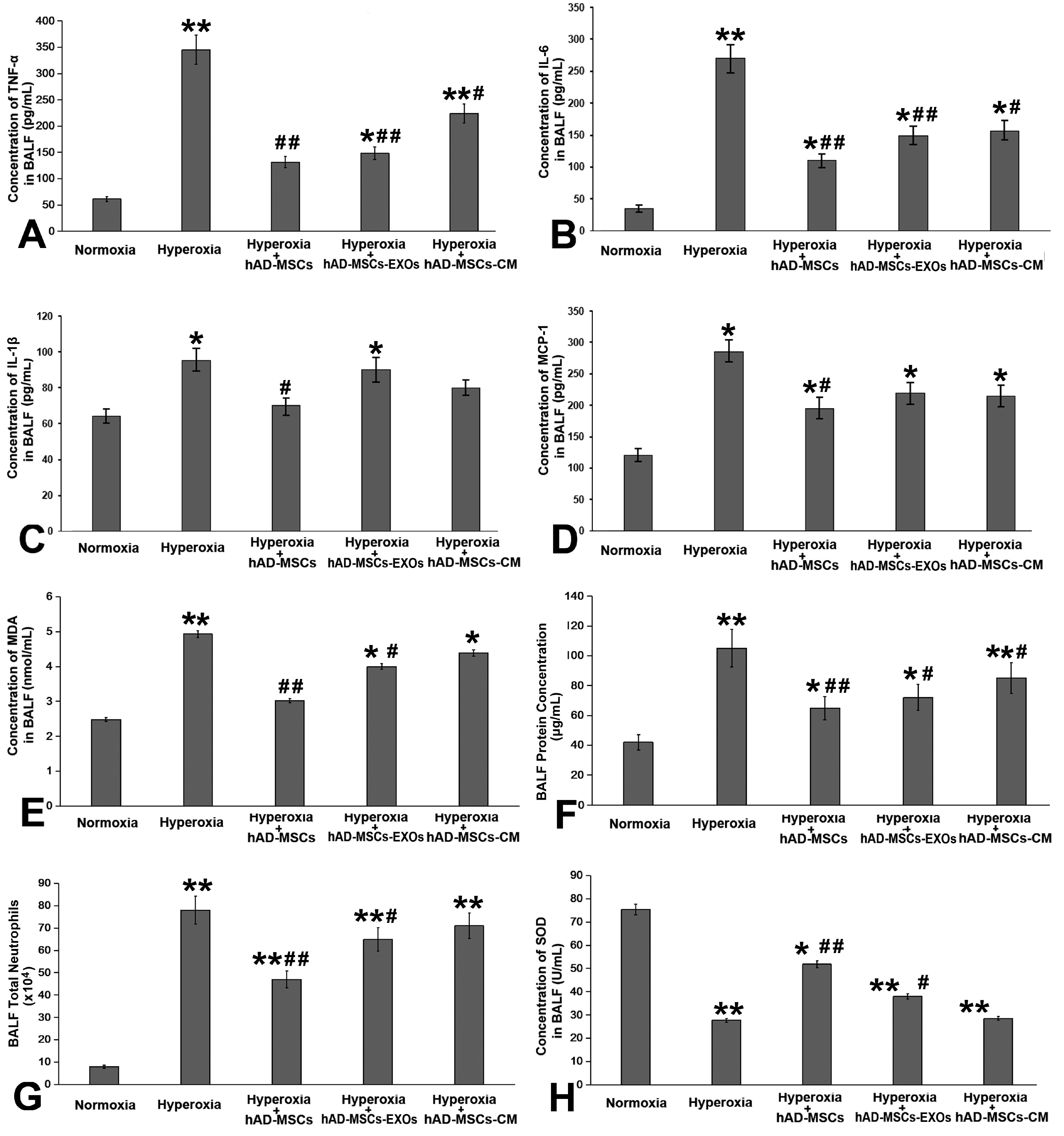
Bronchopulmonary dysplasia (BPD) has major effects in premature infants. Although previous literature has indicated that mesenchymal stem cells (MSCs) can alleviate lung pathology in BPD newborns and improve the survival rate, few research have been done investigating significantly differentially expressed genes in the lungs before and after MSCs therapy. The aim of this study is to identify differentially expressed genes in lung tissues before and after hAD-MSC treatment.
Methods and Results
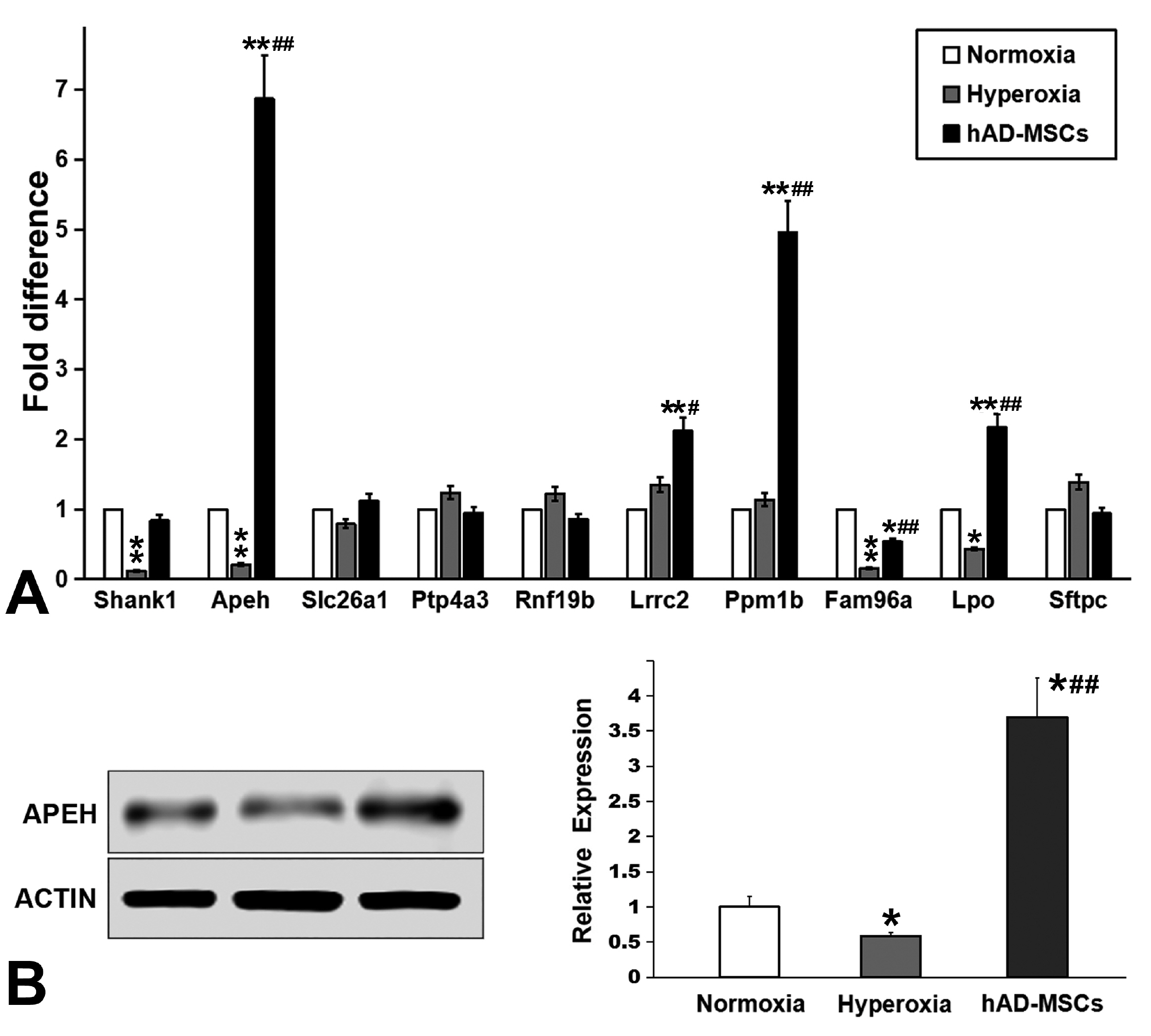
Human amnion-derived MSCs (hAD-MSCs) were cultured and met the MSCs criteria for cell phenotype and multidirectional differentiation. Then we confirmed the size of hAD-MSCs-EXOs and their expressed markers. An intratracheal drip of living cells showed the strongest effect on NHLI compared to cellular secretions or exosomes, both in terms of ameliorating pulmonary edema and reducing inflammatory cell infiltration. Through gene chip hybridization, PCR, and western blotting, acylaminoacyl-peptide hydrolase (APEH) expression was found to be significantly decreased under hyperoxia, and significantly increased after hAD-MSC treatment.
Conclusions
The intratracheal transplantation of hAD-MSCs ameliorated NHLI in neonatal rats through APEH.
Keyword
Figure
Reference
-
References
1. Filippone M, Nardo D, Bonadies L, Salvadori S, Baraldi E. 2019; Update on postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Am J Perinatol. 36(Suppl 2):S58–S62. DOI: 10.1055/s-0039-1691802. PMID: 31238361.
Article2. Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. 2005; Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 115:655–661. DOI: 10.1542/peds.2004-1238. PMID: 15741368.
Article3. Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, Tsai CH, Lin HC. 2016; Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 193:86–95. DOI: 10.1164/rccm.201505-0861OC. PMID: 26351971.
Article4. Kinsella JP, Greenough A, Abman SH. 2006; Bronchopulmonary dysplasia. Lancet. 367:1421–1431. DOI: 10.1016/S0140-6736(06)68615-7. PMID: 16650652.
Article5. Kua KP, Lee SW. 2017; Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br J Clin Pharmacol. 83:180–191. DOI: 10.1111/bcp.13089. PMID: 27526255. PMCID: PMC5338164.
Article6. Li J, Li D, Liu X, Tang S, Wei F. 2012; Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflamm (Lond). 9:33. DOI: 10.1186/1476-9255-9-33. PMID: 22974286. PMCID: PMC3502090.
Article7. Möbius MA, Thébaud B. 2015; Stem cells and their mediators-next generation therapy for bronchopulmonary dysplasia. Front Med (Lausanne). 2:50. DOI: 10.3389/fmed.2015.00050. PMID: 26284246. PMCID: PMC4520239.8. Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, Park WS. 2014; Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 164:966–972.e6. DOI: 10.1016/j.jpeds.2013.12.011. PMID: 24508444.
Article9. Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. 2017; Two-year follow-up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J Pediatr. 185:49–54.e2. DOI: 10.1016/j.jpeds.2017.02.061. PMID: 28341525.
Article10. Díaz-Prado S, Muiños-López E, Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ, Blanco FJ. 2011; Isolation and characterization of mesenchymal stem cells from human amniotic membrane. Tissue Eng Part C Methods. 17:49–59. DOI: 10.1089/ten.tec.2010.0136. PMID: 20673138.
Article11. Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A, Wengler GS, Parolini O. 2007; Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med. 1:296–305. DOI: 10.1002/term.40. PMID: 18038420.
Article12. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. 2004; Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 103:1669–1675. DOI: 10.1182/blood-2003-05-1670. PMID: 14576065.
Article13. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. 2007; Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 179:1855–1863. DOI: 10.4049/jimmunol.179.3.1855. PMID: 17641052.
Article14. Chou HC, Li YT, Chen CM. 2016; Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am J Transl Res. 8:342–353. PMID: 27158330. PMCID: PMC4846887.15. Shen C, Lie P, Miao T, Yu M, Lu Q, Feng T, Li J, Zu T, Liu X, Li H. 2015; Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol Med Rep. 12:20–30. DOI: 10.3892/mmr.2015.3409. PMID: 25739039. PMCID: PMC4438972.
Article16. Devaney J, Horie S, Masterson C, Elliman S, Barry F, O'Brien T, Curley GF, O'Toole D, Laffey JG. 2015; Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 70:625–635. DOI: 10.1136/thoraxjnl-2015-206813. PMID: 25986435.
Article17. Huang B, Cheng X, Wang H, Huang W, la Ga Hu Z, Wang D, Zhang K, Zhang H, Xue Z, Da Y, Zhang N, Hu Y, Yao Z, Qiao L, Gao F, Zhang R. 2016; Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 14:45. DOI: 10.1186/s12967-016-0792-1. PMID: 26861623. PMCID: PMC4746907.
Article18. Xing L, Cui R, Peng L, Ma J, Chen X, Xie RJ, Li B. 2014; Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res Ther. 5:101. DOI: 10.1186/scrt489. PMID: 25145540. PMCID: PMC4159523.
Article19. Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, Yu T, Wang CM, An G, Sha WH, Chen QK. 2015; Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep. 5:8718. DOI: 10.1038/srep08718. PMID: 25732721. PMCID: PMC4346809.
Article20. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. 2009; Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5:54–63. DOI: 10.1016/j.stem.2009.05.003. PMID: 19570514. PMCID: PMC4154377.
Article21. Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. 2003; Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 101:2999–3001. DOI: 10.1182/blood-2002-06-1830. PMID: 12480709.
Article22. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. 2001; The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 169:12–20. DOI: 10.1159/000047856. PMID: 11340257.
Article23. Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. 2016; Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 14:1681–1692. DOI: 10.3892/mmr.2016.5416. PMID: 27357811.
Article24. Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. 2005; Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 106:4057–4065. DOI: 10.1182/blood-2005-03-1004. PMID: 16118325.
Article25. Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R, Reisner Y. 2009; Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 27:2865–2874. DOI: 10.1002/stem.217. PMID: 19750539.
Article26. Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, Lee MS. 2016; Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 216:173–185. DOI: 10.1016/j.ijcard.2016.04.061. PMID: 27156061.
Article27. H Huang L, Ma W, Ma Y, Feng D, Chen H, Cai B. 2015; Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int J Biol Sci. 11:238–245. DOI: 10.7150/ijbs.10725. PMID: 25632267. PMCID: PMC4308409.
Article28. Xu S, Liu C, Ji HL. 2019; Concise review: therapeutic potential of the mesenchymal stem cell derived secretome and extracellular vesicles for radiation-induced lung injury: progress and Hypotheses. Stem Cells Transl Med. 8:344–354. DOI: 10.1002/sctm.18-0038. PMID: 30618085. PMCID: PMC6431606.
Article29. Schoefinius JS, Brunswig-Spickenheier B, Speiseder T, Krebs S, Just U, Lange C. 2017; Mesenchymal stromal cell-derived extracellular vesicles provide long-term survival after total body irradiation without additional hematopoietic stem cell support. Stem Cells. 35:2379–2389. DOI: 10.1002/stem.2716. PMID: 29024236.
Article30. Lou G, Chen Z, Zheng M, Liu Y. 2017; Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 49:e346. DOI: 10.1038/emm.2017.63. PMID: 28620221. PMCID: PMC5519012.
Article31. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. 2015; Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 3:24–32. DOI: 10.1016/S2213-2600(14)70291-7. PMID: 25529339. PMCID: PMC4297579.
Article32. Simones AA, Beisang DJ, Panoskaltsis-Mortari A, Roberts KD. 2018; Mesenchymal stem cells in the pathogenesis and treatment of bronchopulmonary dysplasia: a clinical review. Pediatr Res. 83:308–317. DOI: 10.1038/pr.2017.237. PMID: 28945702. PMCID: PMC5895100.
Article33. Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. 2009; Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 18:869–886. DOI: 10.3727/096368909X471189. PMID: 19500472.
Article34. Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, Emery D, Bodiga S, Eaton F, Péault B, Mosca F, Lazzari L, Thébaud B. 2013; Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 68:475–484. DOI: 10.1136/thoraxjnl-2012-202323. PMID: 23212278.
Article35. Wu Q, Chong L, Shao Y, Chen S, Li C. 2019; Lipoxin A4 reduces hyperoxia-induced lung injury in neonatal rats through PINK1 signaling pathway. Int Immunopharmacol. 73:414–423. DOI: 10.1016/j.intimp.2019.05.046. PMID: 31152979.
Article36. Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. 2004; Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med. 36:782–801. DOI: 10.1016/j.freeradbiomed.2003.12.007. PMID: 14990357.
Article37. Oncel MY, Yurttutan S, Alyamac Dizdar E, Gokce IK, Gonul II, Topal T, Canpolat FE, Dilmen U. 2016; Beneficial effect of etanercept on hyperoxic lung injury model in neonatal rats. J Invest Surg. 29:1–5. DOI: 10.3109/08941939.2015.1034898. PMID: 26305557.
Article38. Chen C, Shi L, Li Y, Wang X, Yang S. 2016; Disease-specific dynamic biomarkers selected by integrating inflammatory mediators with clinical informatics in ARDS patients with severe pneumonia. Cell Biol Toxicol. 32:169–184. DOI: 10.1007/s10565-016-9322-4. PMID: 27095254. PMCID: PMC4882347.
Article39. Zhang H, Fang J, Su H, Yang M, Lai W, Mai Y, Wu Y. 2012; Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant. 16:589–598. DOI: 10.1111/j.1399-3046.2012.01709.x. PMID: 22738184.
Article40. Zhang X, Wang H, Shi Y, Peng W, Zhang S, Zhang W, Xu J, Mei Y, Feng Z. 2012; Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 36:589–594. DOI: 10.1042/CBI20110447. PMID: 22339670.
Article41. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. 2010; A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 5:e10088. DOI: 10.1371/journal.pone.0010088. PMID: 20436665. PMCID: PMC2859930.
Article42. Romieu-Mourez R, François M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. 2009; Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 182:7963–7973. DOI: 10.4049/jimmunol.0803864. PMID: 19494321.
Article43. Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y. 2012; Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 19:1505–1513. DOI: 10.1038/cdd.2012.26. PMID: 22421969. PMCID: PMC3422473.
Article44. Syed MA, Bhandari V. 2013; Hyperoxia exacerbates postnatal inflammation-induced lung injury in neonatal BRP-39 null mutant mice promoting the M1 macrophage phenotype. Mediators Inflamm. 2013:457189. DOI: 10.1155/2013/457189. PMID: 24347826. PMCID: PMC3855965.
Article45. Manferdini C, Paolella F, Gabusi E, Gambari L, Piacentini A, Filardo G, Fleury-Cappellesso S, Barbero A, Murphy M, Lisignoli G. 2017; Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis Cartilage. 25:1161–1171. DOI: 10.1016/j.joca.2017.01.011. PMID: 28153787.
Article46. Chiossone L, Conte R, Spaggiari GM, Serra M, Romei C, Bellora F, Becchetti F, Andaloro A, Moretta L, Bottino C. 2016; Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells. 34:1909–1921. DOI: 10.1002/stem.2369. PMID: 27015881.
Article47. Magatti M, Vertua E, De Munari S, Caro M, Caruso M, Silini A, Delgado M, Parolini O. 2017; Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J Tissue Eng Regen Med. 11:2895–2911. DOI: 10.1002/term.2193. PMID: 27396853. PMCID: PMC5697700.
Article48. Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, Zhao X, Lu C. 2019; Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res. 38:62. DOI: 10.1186/s13046-019-1027-0. PMID: 30736829. PMCID: PMC6367822.
Article49. Dave M, Jaiswal P, Cominelli F. 2017; Mesenchymal stem/stromal cell therapy for inflammatory bowel disease: an updated review with maintenance of remission. Curr Opin Gastroenterol. 33:59–68. DOI: 10.1097/MOG.0000000000000327. PMID: 28134690.50. Chen D, Dou QP. 2010; The ubiquitin-proteasome system as a prospective molecular target for cancer treatment and prevention. Curr Protein Pept Sci. 11:459–470. DOI: 10.2174/138920310791824057. PMID: 20491623. PMCID: PMC3306609.
Article51. Iyer SS, Jones DP, Brigham KL, Rojas M. 2009; Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am J Respir Cell Mol Biol. 40:90–98. DOI: 10.1165/rcmb.2007-0447OC. PMID: 18664641. PMCID: PMC2606950.
Article52. Tahara M, Nakayama M, Jin MB, Fujita M, Suzuki T, Taniguchi M, Shimamura T, Furukawa H, Todo S. 2005; A radical scavenger, edaravone, protects canine kidneys from ischemia-reperfusion injury after 72 hours of cold preservation and autotransplantation. Transplantation. 80:213–221. DOI: 10.1097/01.TP.0000165092.07375.C9. PMID: 16041266.
Article53. Wang J, Dong W. 2018; Oxidative stress and bronchopulmonary dysplasia. Gene. 678:177–183. DOI: 10.1016/j.gene.2018.08.031. PMID: 30098433.
Article54. Cadenas S. 2018; ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 117:76–89. DOI: 10.1016/j.freeradbiomed.2018.01.024. PMID: 29373843.
Article55. Sies H. 2017; Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11:613–619. DOI: 10.1016/j.redox.2016.12.035. PMID: 28110218. PMCID: PMC5256672.
Article56. Tiganis T. 2011; Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol Sci. 32:82–89. DOI: 10.1016/j.tips.2010.11.006. PMID: 21159388.
Article57. Abele D, Puntarulo S. 2004; Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A Mol Integr Physiol. 138:405–415. DOI: 10.1016/j.cbpb.2004.05.013. PMID: 15369829.
Article58. García-Rojo G, Gámiz F, Ampuero E, Rojas-Espina D, Sandoval R, Rozas C, Morales B, Wyneken U, Pancetti F. 2017; In vivo sub-chronic treatment with dichlorvos in young rats promotes synaptic plasticity and learning by a mechanism that involves acylpeptide hydrolase instead of acetylcholinesterase inhibition. Correlation with endogenous β-amyloid levels. Front Pharmacol. 8:483. DOI: 10.3389/fphar.2017.00483. PMID: 28790916. PMCID: PMC5524899.59. Zeng Z, Rulten SL, Breslin C, Zlatanou A, Coulthard V, Caldecott KW. 2017; Acylpeptide hydrolase is a component of the cellular response to DNA damage. DNA Repair (Amst). 58:52–61. DOI: 10.1016/j.dnarep.2017.08.008. PMID: 28866241.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-Term (Postnatal Day 70) Outcome and Safety of Intratracheal Transplantation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Neonatal Hyperoxic Lung Injury
- Fetal Alveolar Type II Cell Injury Induced by Short-term Exposure to Hyperoxia
- Intratracheal Administration of Endotoxin Attenuates Hyperoxia-Induced Lung Injury in Neonatal Rats
- Cell Division Cycle 2 Protects Neonatal Rats Against Hyperoxia-Induced Bronchopulmonary Dysplasia
- The Effects of Bone Marrow-Derived Mesenchymal Stem Cells on Alveolarization Inhibition Induced by Hyperoxia in Neonatal Rat