Anat Cell Biol.
2020 Jun;53(2):183-193. 10.5115/acb.19.205.
Ficus exasperata Vahl leaves extract attenuates motor deficit in vanadium-induced parkinsonism mice
- Affiliations
-
- 1Department of Anatomy, College of Medicine and Health Sciences, Afe Babalola University, Ado Ekiti, Nigeria
- 2Department of Anatomy, Faculty of Basic Medical Sciences, College of Medicine, Olabisi Onabanjo University, Ago Iwoye, Nigeria
- KMID: 2503448
- DOI: http://doi.org/10.5115/acb.19.205
Abstract
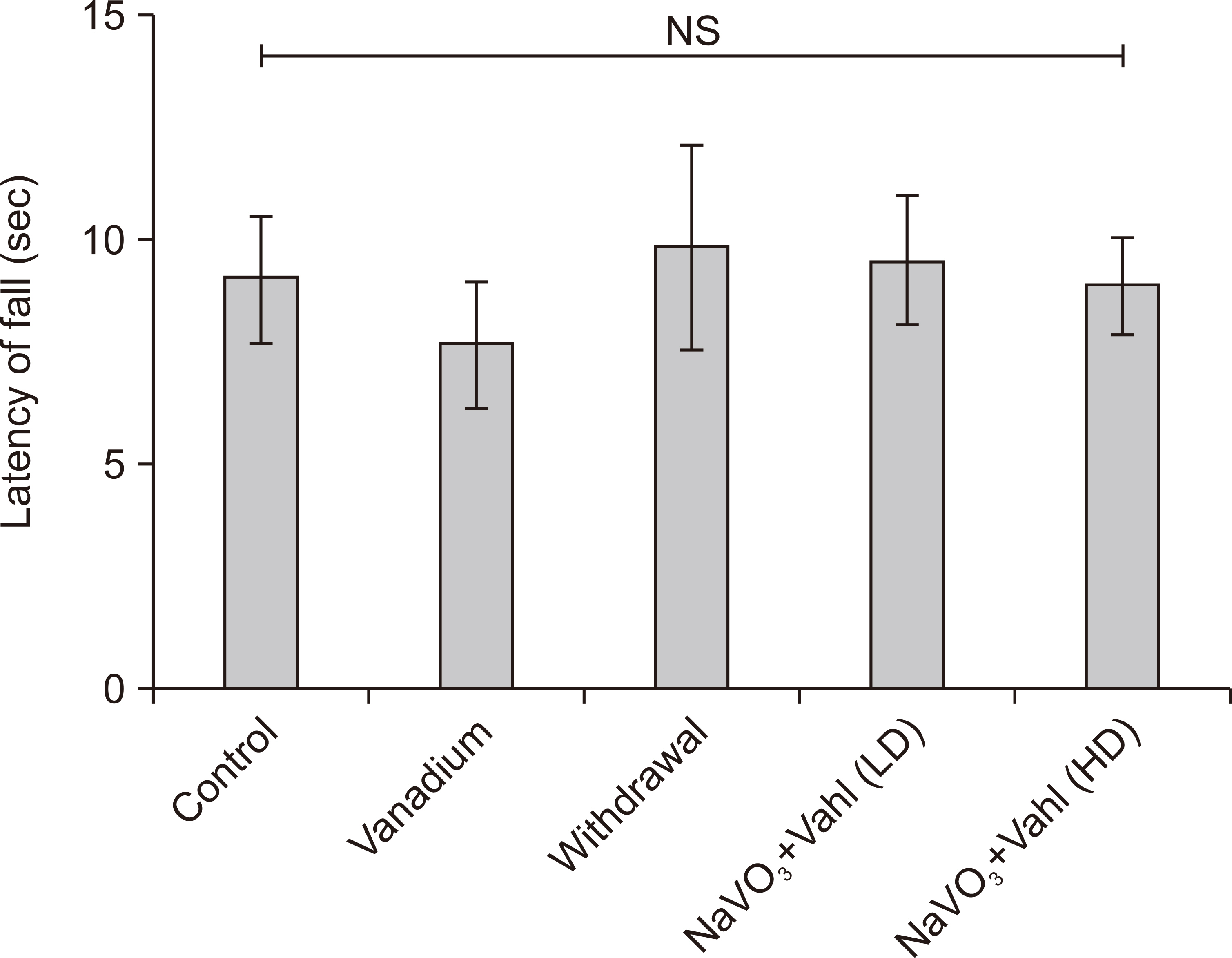
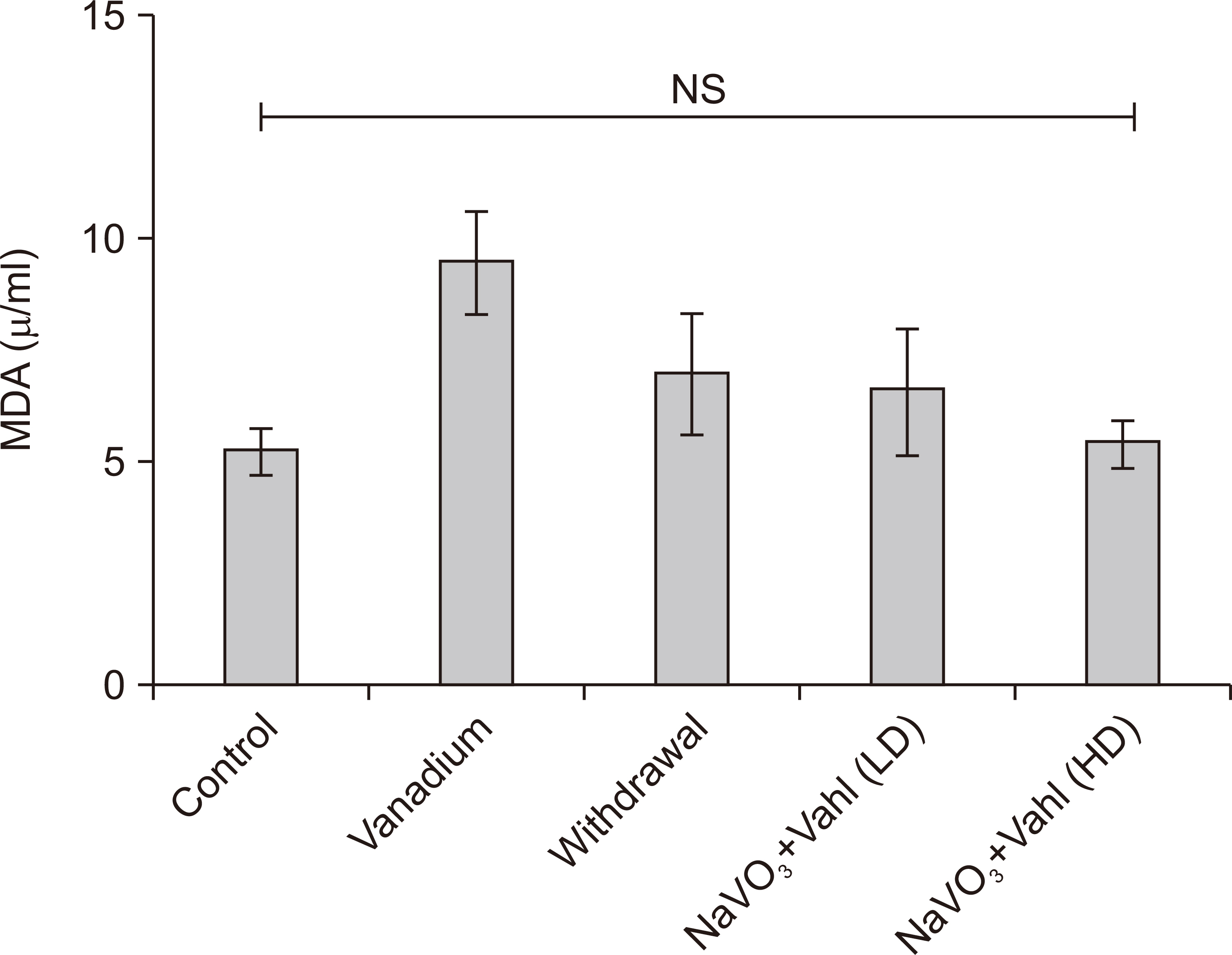
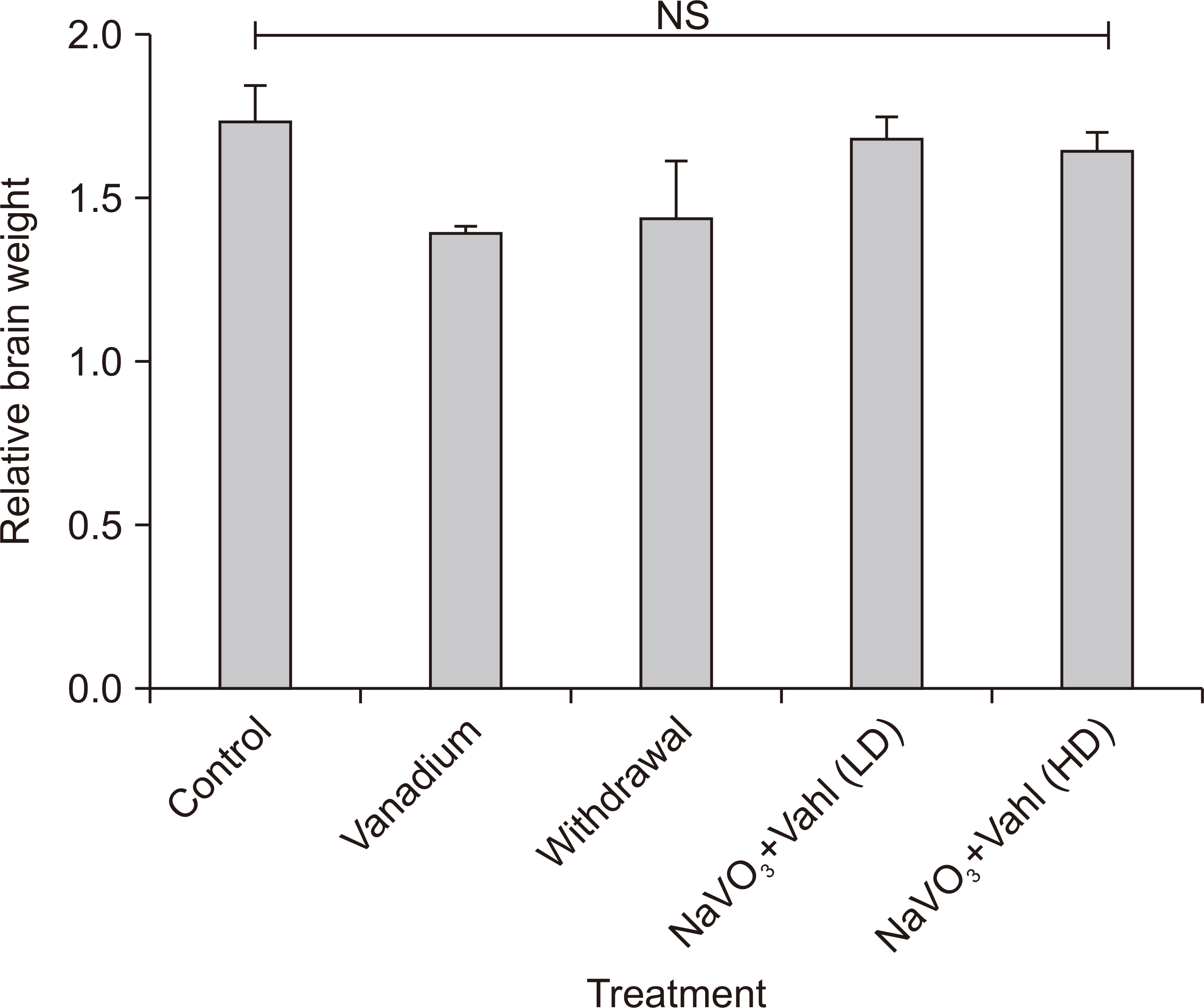
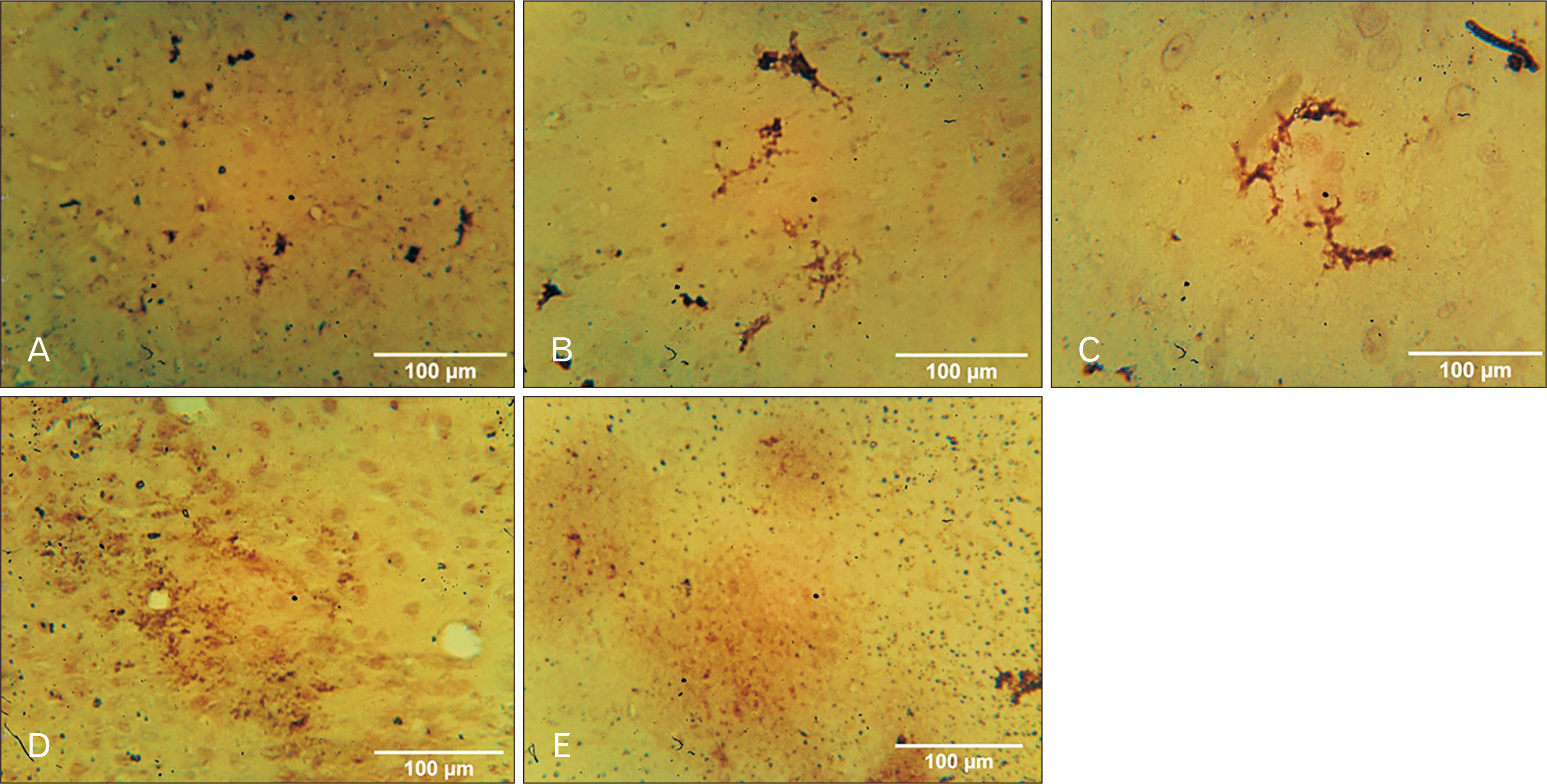
- Medicinal herbs have played significant roles in the treatment of various diseases in humans and animals. Sodium metavanadate is a potentially toxic environmental pollutant that induces oxidative damage, neurological disorder, Parkinsonism and Parkinson-like disease upon excessive exposure. This study is designed to investigate the impact of saponin fraction of Ficus exasperata Vahl leaf extract (at 50 and 100 mg/kg body weight for 14 days at different animal groupings) on vanadium treated mice. Animals were randomly grouped into five groups. Control (normal saline), NaVO3 (10 mg/kg for 7 days), withdrawal group, NaVO3+Vahl (low dose) and NaVO3+Vahl (high dose). The animals were screened for motor coordination using rotarod and PBTs and a post mortem study was conducted by quantitatively assessing the markers of oxidative stress such as lipid peroxidation, catalase, glutathione activities, and also through immunohistochemistry via glia fibrillary acidic protein, tyrosine hydroxylase and dopamine transporter to study the integrity of astrocytes and dopaminergic neurons of the substantia nigra (SNc). Vanadium-exposed group showed a decreased motor activity on the neurobehavioural tests as well as an increase in markers of oxidative stress. Saponin fraction of F. exasperata Vahl leaves extract produced a statistically significant motor improvement which may be due to high antioxidant activities of saponin, thereby providing an ameliorative effect on the histoarchitecture of the SNc. It can be inferred that the saponin fraction of F. exasperata Vahl leaves extract to possesses ameliorative, motor-enhancing and neurorestorative benefit on motor deficit in vanadium-induced parkinsonism mice.
Figure
Cited by 1 articles
-
Tamarindus indica ameliorates behavioral and cytoarchitectural changes in the cerebellar cortex following prenatal aluminum chloride exposure in Wistar rats
Ibe Michael Usman, Samuel Sunday Adebisi, Sunday Abraham Musa, Ibrahim Abdullahi Iliya, Victor Bassey Archibong, Ann Monima Lemuel, Keneth Iceland Kasozi
Anat Cell Biol. 2022;55(3):320-329. doi: 10.5115/acb.22.033.
Reference
-
1. Hsu HW, Bondy SC, Kitazawa M. 2018; Environmental and dietary exposure to copper and its cellular mechanisms linking to Alzheimer's disease. Toxicol Sci. 163:338–45. DOI: 10.1093/toxsci/kfy025. PMID: 29409005. PMCID: PMC5974786.
Article2. Liu J, Lewis G. 2014; Environmental toxicity and poor cognitive outcomes in children and adults. J Environ Health. 76:130–8. PMID: 24645424. PMCID: PMC4247328.3. Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. 2001; Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 56:8–13. DOI: 10.1212/WNL.56.1.8. PMID: 11148228.
Article4. Fafure AA, Adekeye AO, Enye LA, Tijani AA, Ajao MM, Edem EE. 2018; Ficus exasperata vahl improves manganese-induced neurotoxicity and motor dysfunction in mice. Anat J Afr. 7:1206–19.
Article5. Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. 2009; Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson's disease. Toxicol Appl Pharmacol. 240:273–85. DOI: 10.1016/j.taap.2009.07.025. PMID: 19646462. PMCID: PMC2753722.
Article6. Moskalyk RR, Alfantazi AM. 2003; Processing of vanadium: a review. Miner Eng. 16:793–805. DOI: 10.1016/S0892-6875(03)00213-9.
Article7. Amorim FA, Welz B, Costa AC, Lepri FG, Vale MG, Ferreira SL. 2007; Determination of vanadium in petroleum and petroleum products using atomic spectrometric techniques. Talanta. 72:349–59. DOI: 10.1016/j.talanta.2006.12.015. PMID: 19071624.
Article8. Olopade JO, Connor JR. 2011; Vanadium and neurotoxicity: a review. Curr Top Toxicol. 7:33–9.9. Fortoul TI, Rodriguez-Lara V, González-Villalva A, Rojas-Lemus M, Cano-Gutiérrez G, Ustarroz-Cano M, Colín-Barenque L, Bizarro-Nevares P, García-Pealez I, Montaño LF, Jimenez-Martinez RS, Lopez-Valdez N, Ruiz-Guerrero ML, Meléndez-García NA, García-Ibarra FA, Martínez-Baez V, Zapata Alfaro D, Muñiz-Rivera-Cambas A, López-Zepeda LS, Quezada-Maldonado EM, Cervantes-Yépez S. 2014; Inhalation of vanadium pentoxide and its toxic effects in a mouse model. Inorganica Chim Acta. 420:8–15. DOI: 10.1016/j.ica.2014.03.027.
Article10. Roy A, Jauhari N, Bharadvaja N. Akhtar MS, Swamy MK, editors. 2018. Medicinal plants as a potential source of chemopreventive agents. Anticancer plants: natural products and biotechnological implements. Springer;Singapore: p. 109. DOI: 10.1007/978-981-10-8064-7_6.
Article11. Odiba PA, Yusuf D, Ali E, Yusuf MI, John E. 2012; Effect of aqueous extract of Ficus exasperata leaf on the body weight and haematological parameters of Wistar rats. J Appl Sci Environ. 3:80–3.12. Ahmed F, Toume K, Ohtsuki T, Rahman M, Sadhu SK, Ishibashi M. 2011; Cryptolepine, isolated from Sida acuta, sensitizes human gastric adenocarcinoma cells to TRAIL-induced apoptosis. Phytother Res. 25:147–50. DOI: 10.1002/ptr.3219. PMID: 20623717.
Article13. Ijeh , Ukweni AI. 2007; Acute effect of administration of ethanol extracts of Ficus exasperata vahl on kidney function in albino rats. II. J Med Plant Res. 1:27–9.14. Akah PA, Orisakwe OE, Gamaniel KS, Shittu A. 1998; Evaluation of Nigerian traditional medicines: II. effects of some Nigerian folk remedies on peptic ulcer. J Ethnopharmacol. 62:123–7. DOI: 10.1016/S0378-8741(98)00060-9. PMID: 9741884.
Article15. Ogunleye RF. 2011; Effectiveness of the leaf powder of Ficus exasperata Vahl. (Moraceae) in suppressing thepopulation of two major storage insect pests. Cont J Biol Sci. 4:6–11.16. Ayinde BA, Omogbai EK, Amaechina FC. 2007; Pharmacognosy and hypotensive evaluation of Ficus exasperata Vahl (Moraceae) leaf. Acta Pol Pharm. 64:543–6. PMID: 18323249.17. Devbhuti D, Gupta JK, Devbhuti P, Bose A. 2009; Phytochemical and acute toxicity study on Tinospora tomentosa Miers. Acta Pol Pharm. 66:89–92.18. Tijjani MB, Bello IA, Aliyu AB, Olurishe T, Maidawa SM, Habila JD, Balogun EO. 2009; Phytochemical and antibacterial studies of root extract of Cochlospermum tinctorium A. Rich. (Cochlospermaceae). Res J Med Plant. 3:16–22. DOI: 10.3923/rjmp.2009.16.22.
Article19. Sparg SG, Light ME, van Staden J. 2004; Biological activities and distribution of plant saponins. J Ethnopharmacol. 94:219–43. DOI: 10.1016/j.jep.2004.05.016. PMID: 15325725.
Article20. Nandakumar V, Singh T, Katiyar SK. 2008; Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 269:378–87. DOI: 10.1016/j.canlet.2008.03.049. PMID: 18457915. PMCID: PMC2562893.
Article21. Adekeye AO, Adumah C, Fafure AA, Ajao M, Sabiu S, Shallie P, Adefule AK. 2018; Evaluation of the effects of gutenbergia nigritana leaves extract on cerebellum of adult mice and its implication on manganese toxicity. World J Pharm Pharm Sci. 7:55–68. DOI: 10.1016/j.ibror.2019.09.046.22. Mustapha O, Oke B, Offen N, Sirén AL, Olopade J. 2014; Neurobehavioral and cytotoxic effects of vanadium during oligodendrocyte maturation: a protective role for erythropoietin. Environ Toxicol Pharmacol. 38:98–111. DOI: 10.1016/j.etap.2014.05.001. PMID: 24927405.
Article23. Folarin O, Olopade F, Onwuka S, Olopade J. 2016; Memory deficit recovery after chronic vanadium exposure in mice. Oxid Med Cell Longev. 2016:4860582. DOI: 10.1155/2016/4860582. PMID: 26962395. PMCID: PMC4745327.
Article24. Carter RJ, Morton J, Dunnett SB. 2001; Motor coordination and balance in rodents. Curr Protoc Neurosci. Chapter 8:Unit 8. 12. DOI: 10.1002/0471142301.ns0812s15. PMID: 18428540.
Article25. Zwolak I. 2014; Vanadium carcinogenic, immunotoxic and neurotoxic effects: a review of in vitro studies. Toxicol Mech Methods. 24:1–12. DOI: 10.3109/15376516.2013.843110. PMID: 24147425.26. Folarin OR, Snyder AM, Peters DG, Olopade F, Connor JR, Olopade JO. 2017; Brain metal distribution and neuro-inflammatory profiles after chronic vanadium administration and withdrawal in mice. Front Neuroanat. 11:58. DOI: 10.3389/fnana.2017.00058. PMID: 28790895. PMCID: PMC5524677.
Article27. Igado OO, Olopade JO, Adesida A, Aina OO, Farombi EO. 2012; Morphological and biochemical investigation into the possible neuroprotective effects of kolaviron (Garcinia kola bioflavonoid) on the brains of rats exposed to vanadium. Drug Chem Toxicol. 35:371–80. DOI: 10.3109/01480545.2011.630005. PMID: 22288905.28. Fatola OI, Olaolorun FA, Olopade FE, Olopade JO. 2019; Trends in vanadium neurotoxicity. Brain Res Bull. 145:75–80. DOI: 10.1016/j.brainresbull.2018.03.010. PMID: 29577939.
Article29. Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. 1989; Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 52:381–9. DOI: 10.1111/j.1471-4159.1989.tb09133.x. PMID: 2911023.
Article30. Nissanka N, Moraes CT. 2018; Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 592:728–42. DOI: 10.1002/1873-3468.12956. PMID: 29281123. PMCID: PMC6942696.
Article31. Shi X, Dalal NS. 1993; Vanadate-mediated hydroxyl radical generation from superoxide radical in the presence of NADH: Haber-Weiss vs Fenton mechanism. Arch Biochem Biophys. 307:336–41. DOI: 10.1006/abbi.1993.1597. PMID: 8274019.
Article32. Lee S, Li J, Zhou X, Yin J, Yoon J. 2018; Recent progress on the development of glutathione (GSH) selective fluorescent and colorimetric probes. Coord Chem Rev. 366:29–68. DOI: 10.1016/j.ccr.2018.03.021.
Article33. Monleau M, De Méo M, Frelon S, Paquet F, Donnadieu-Claraz M, Duménil G, Chazel V. 2006; Distribution and genotoxic effects after successive exposure to different uranium oxide particles inhaled by rats. Inhal Toxicol. 18:885–94. DOI: 10.1080/08958370600822524. PMID: 16864406.
Article34. Ooms M, Celen S, De Hoogt R, Lenaerts I, Liebregts J, Vanhoof G, Langlois X, Postnov A, Koole M, Verbruggen A, Van Laere K, Bormans G. 2017; Striatal phosphodiesterase 10A availability is altered secondary to chronic changes in dopamine neurotransmission. EJNMMI Radiopharm Chem. 1:3. DOI: 10.1186/s41181-016-0005-5. PMID: 29564380. PMCID: PMC5843803.
Article35. Schmitt KC, Reith ME. 2010; Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 1187:316–40. DOI: 10.1111/j.1749-6632.2009.05148.x. PMID: 20201860.36. Chinyere NH. 2012. Studies on anti-inflammatory properties of the leaf extracts of ficus exasperata Vahl (Moraceae) [Doctoral dissertation]. University of Nigeria Nsukka;Nsukka:37. Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, Levey AI. 1997; Immunochemical analysis of dopamine transporter protein in Parkinson's disease. Ann Neurol. 41:530–9. DOI: 10.1002/ana.410410417. PMID: 9124811.
Article38. Fang SC, Hsu CL, Yen GC. 2008; Anti-inflammatory effects of phenolic compounds isolated from the fruits of Artocarpus heterophyllus. J Agric Food Chem. 56:4463–8. DOI: 10.1021/jf800444g. PMID: 18500810.39. Heller JP, Rusakov DA. 2015; Morphological plasticity of astroglia: understanding synaptic microenvironment. Glia. 63:2133–51. DOI: 10.1002/glia.22821. PMID: 25782611. PMCID: PMC4737250.
Article40. Garcia GB, Biancardi ME, Quiroga AD. 2005; Vanadium (V)-induced neurotoxicity in the rat central nervous system: a histo-immunohistochemical study. Drug Chem Toxicol. 28:329–44. DOI: 10.1081/DCT-200064496. PMID: 16051558.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial Activity of Methanol Extract from Ficus carica Leaves Against Oral Bacteria
- Induction of apoptosis by methanol extracts of Ficus carica L. in FaDu human hypopharynx squamous carcinoma cells
- Antidepressant-like Effect of Kaempferol and Quercitirin, Isolated from Opuntia ficus-indica var. saboten
- Effect of Antimicrobiotic of Opuntia ficus-indica on Surface Disinfection
- Association between extract from fruit of Opuntia ficus-indica and streptozotocin-induced diabetic rats