J Korean Neurosurg Soc.
2020 Mar;63(2):248-260. 10.3340/jkns.2019.0046.
Clinical Uses of Diffusion Tensor Imaging Fiber Tracking Merged Neuronavigation with Lesions Adjacent to Corticospinal Tract : A Retrospective Cohort Study
- Affiliations
-
- 1Department of Neurosurgery, Shengjing Hospital of China Medical University, Shenyang, China
- 2Liaoning Clinical Medical Research Center in Nervous System Disease, Liaoning, China
- 3Liaoning Key Laboratory of Neuro-Oncology, Liaoning, China
- 4Department of Radiology, Shengjing Hospital of China Medical University, Shenyang, China
- KMID: 2501711
- DOI: http://doi.org/10.3340/jkns.2019.0046
Abstract
Objective
: To investigate the efficiency of diffusion tensor imaging (DTI) fiber-tracking based neuronavigation and assess its usefulness in the preoperative surgical planning, prognostic prediction, intraoperative course and outcome improvement.
Methods
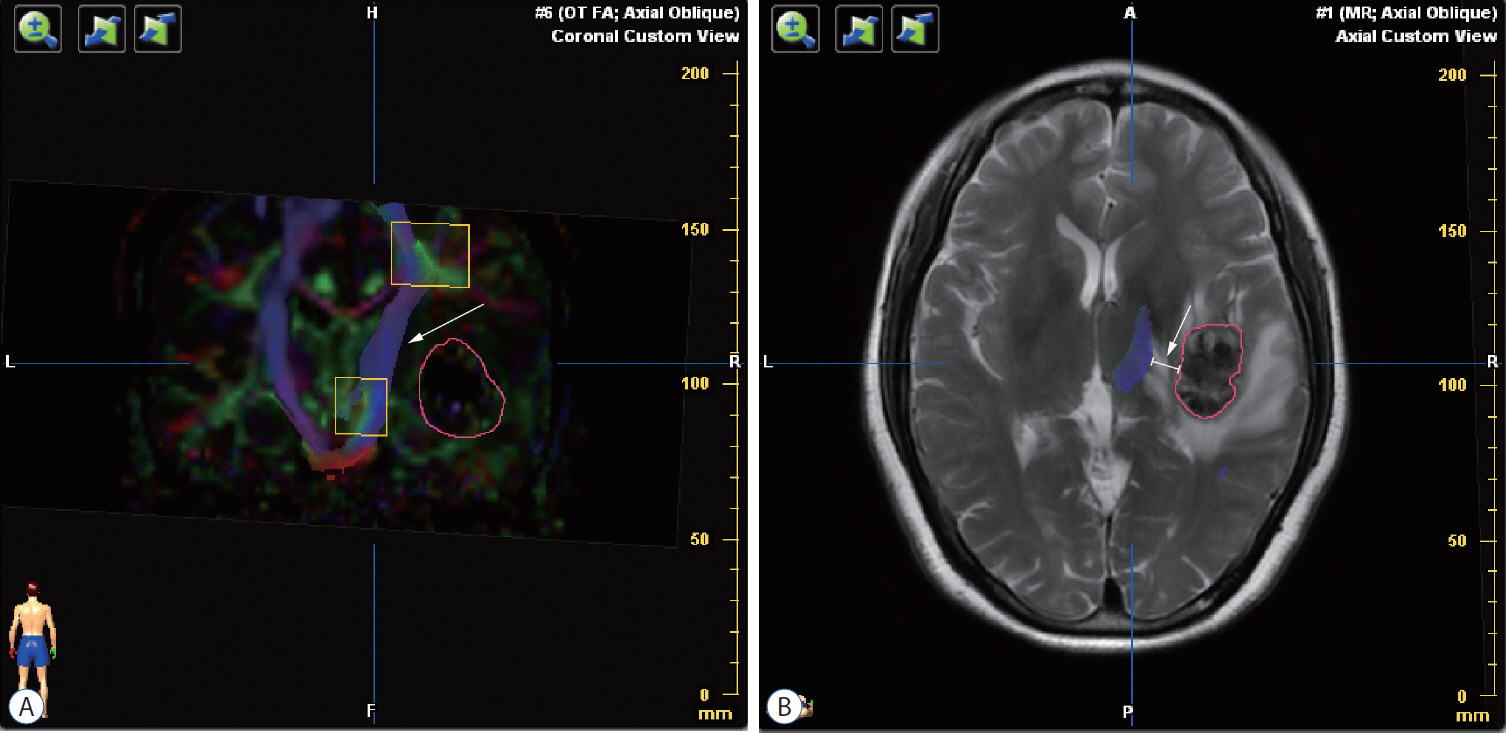
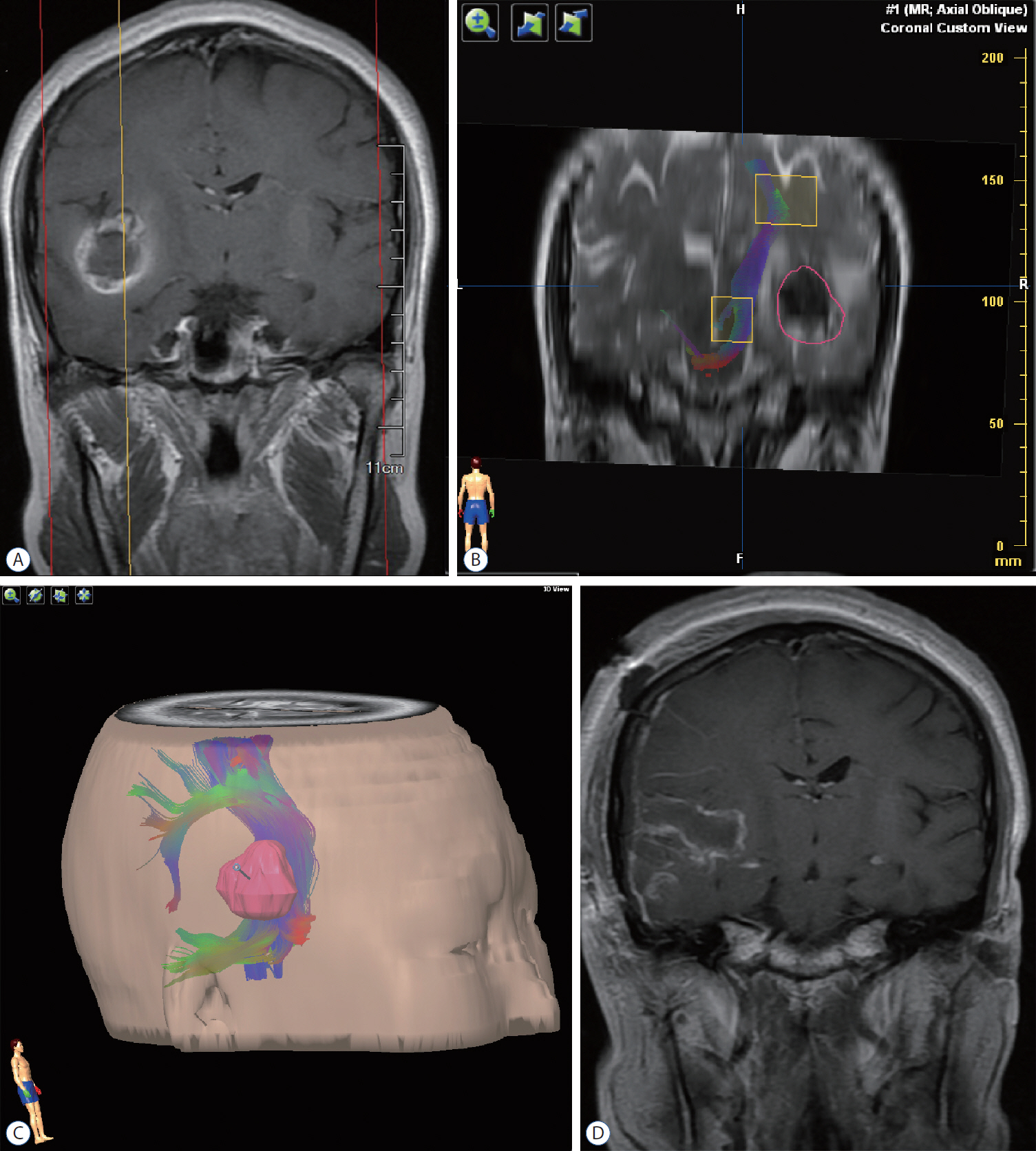
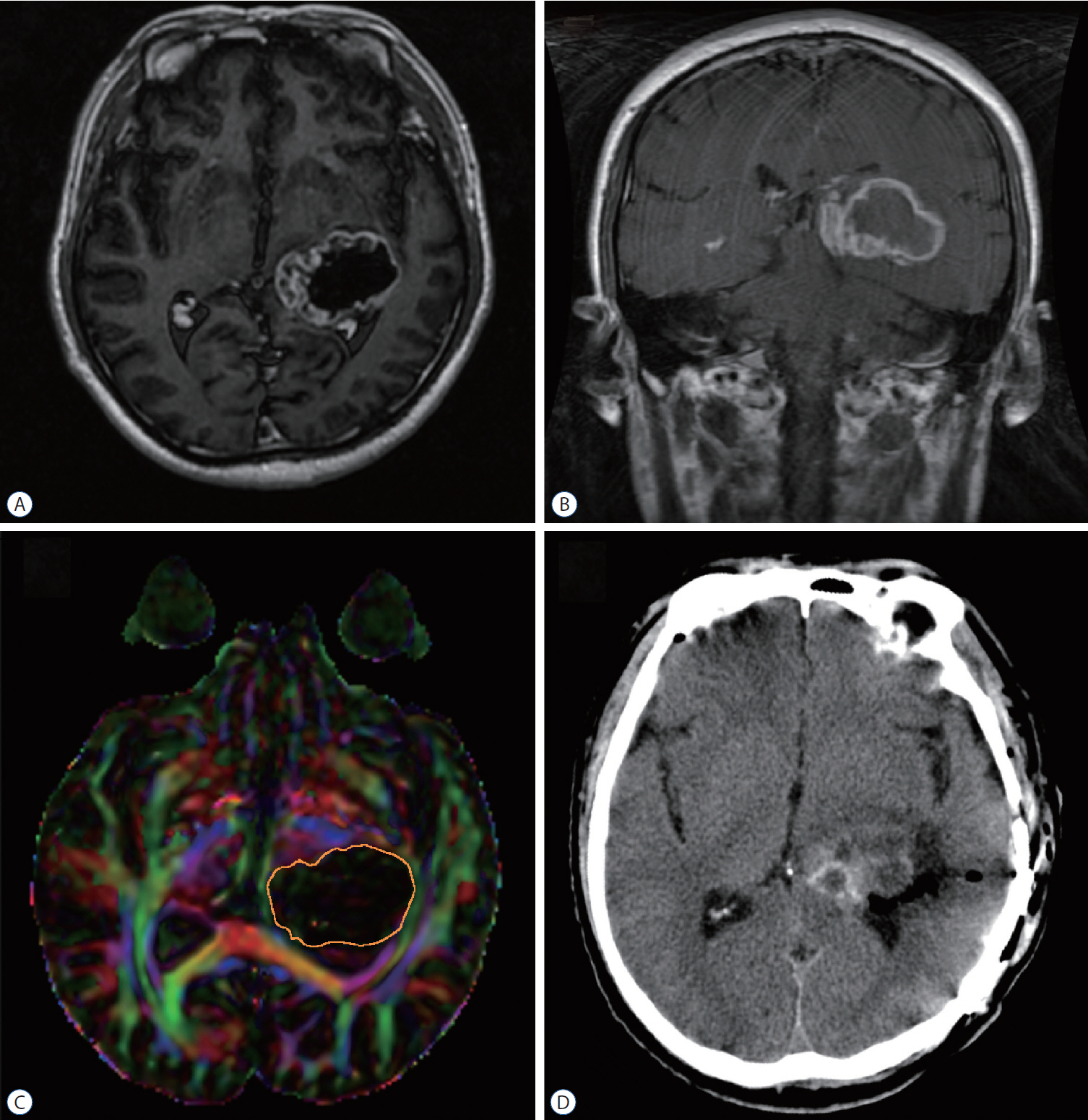
: Seventeen patients with cerebral masses adjacent to corticospinal tract (CST) were given standard magnetic resonance imaging and DTI examination. By incorporation of DTI data, the relation between tumor and adjacent white matter tracts was reconstructed and assessed in the neuronavigation system. Distance from tumor border to CST was measured.
Results
: The sub-portion of CST in closest proximity to tumor was found displaced in all patients. The chief disruptive changes were classified as follows : complete interruption, partial interruption, or simple displacement. Partial interruption was evident in seven patients (41.2%) whose lesions were close to cortex. In the other 10 patients (58.8%), delineated CSTs were intact but distorted. No complete CST interruption was identified. Overall, the mean distance from resection border to CST was 6.12 mm (range, 0–21), as opposed to 8.18 mm (range, 2–21) with simple displacement and 2.33 mm (range, 0–5) with partial interruption. The clinical outcomes were analyzed in groups stratified by intervening distances (close, <5 mm; moderated, 5–10 mm; far, >10 mm). For the primary brain tumor patients, the proportion of completely resected tumors increased progressively from close to far grouping (42.9%, 50%, and 100%, respectively). Five patients out of seven (71.4%) experienced new neurologic deficits postoperatively in the close group. At meantime, motor deterioration was found in six cases in the close group. All patients in the far and moderate groups received excellent (modified Rankin Scale [mRS] score, 0–1) or good (mRS score, 2–3) rankings, but only 57.1% of patients in the close group earned good outcome scores.
Conclusion
: DTI fiber tracking based neuronavigation has merit in assessing the relation between lesions and adjacent white matter tracts, allowing prediction of patient outcomes based on lesion-CST distance. It has also proven beneficial in formulating surgical strategies.
Figure
Reference
-
References
1. Andersen O, Hildeman A, Longfils M, Tedeholm H, Skoog B, Tian W, et al. Diffusion tensor imaging in multiple sclerosis at different final outcomes. Acta Neurol Scand. 137:165–173. 2017.
Article2. Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. (1):CD009685. 2014.
Article3. Benveniste R, Germano IM. Evaluation of factors predicting accurate resection of high-grade gliomas by using frameless image-guided stereotactic guidance. Neurosurg Focus. 14:e5. 2003.
Article4. Bonilha L, Gleichgerrcht E, Fridriksson J, Rorden C, Breedlove JL, Nesland T, et al. Reproducibility of the structural brain connectome derived from diffusion tensor imaging. PLoS One. 10:e0135247. 2015.
Article5. Bucci M, Mandelli ML, Berman JI, Amirbekian B, Nguyen C, Berger MS, et al. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. Neuroimage Clin. 3:361–368. 2013.
Article6. Bürgel U, Mädler B, Honey CR, Thron A, Gilsbach J, Coenen VA. Fiber tracking with distinct software tools results in a clear diversity in anatomical fiber tract portrayal. Cent Eur Neurosurg. 70:27–35. 2009.
Article7. Chen Z, Ni P, Zhang J, Ye Y, Xiao H, Qian G, et al. Evaluating ischemic stroke with diffusion tensor imaging. Neurol Res. 30:720–726. 2008.
Article8. Di X, Sui A, Hakim R, Wang M, Warnke JP. Endoscopic minimally invasive neurosurgery: emerging techniques and expanding role through an extensive review of the literature and our own experience - part I: intraendoscopic neurosurgery. Pediatr Neurosurg. 47:315–326. 2011.
Article9. Filler AG, Howe FA, Hayes CE, Kliot M, Winn HR, Bell BA, et al. Magnetic resonance neurography. Lancet. 341:659–661. 1993.
Article10. Fu JL, Liu Y, Li YM, Chang C, Li WB. Use of diffusion tensor imaging for evaluating changes in the microstructural integrity of white matter over 3 years in patients with amnesic-type mild cognitive impairment converting to Alzheimer’s disease. J Neuroimaging. 24:343–348. 2014.
Article11. Garrett M, Consiglieri G, Nakaji P. Transcranial minimally invasive neurosurgery for tumors. Neurosurg Clin N Am. 21:595–605. 2010.
Article12. Golby AJ, Kindlmann G, Norton I, Yarmarkovich A, Pieper S, Kikinis R. Interactive diffusion tensor tractography visualization for neurosurgical planning. Neurosurgery. 68:496–505. 2011.
Article13. Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, et al. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 19:524–536. 2009.
Article14. Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS One. 2:e597. 2007.
Article15. Handsfield GG, Bolsterlee B, Inouye JM, Herbert RD, Besier TF, Fernandez JW. Determining skeletal muscle architecture with Laplacian simulations: a comparison with diffusion tensor imaging. Biomech Model Mechanobiol. 16:1845–1855. 2017.
Article16. Krieg SM, Buchmann NH, Gempt J, Shiban E, Meyer B, Ringel F. Diffusion tensor imaging fiber tracking using navigated brain stimulation--a feasibility study. Acta Neurochir (Wien). 154:555–563. 2012.
Article17. Kunimatsu A, Aoki S, Masutani Y, Abe O, Hayashi N, Mori H, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci. 3:11–17. 2004.
Article18. Liu X, Tian W, Chen H, LoStracco TA, Zhang J, Li MY, et al. Advanced neuroimaging in the evaluation of spinal cord tumors and tumor mimics: diffusion tensor and perfusion-weighted imaging. Semin Ultrasound CT MR. 38:163–175. 2017.
Article19. Ma J, Cheng L, Wang G, Lin S. Surgical management of meningioma of the trigone area of the lateral ventricle. World Neurosurg. 82:757–769. 2014.
Article20. Manjila S, Karhade A, Phi JH, Scott RM, Smith ER. Real-time ultrasoundguided catheter navigation for approaching deep-seated brain lesions: role of intraoperative neurosonography with and without fusion with magnetic resonance imaging. Pediatr Neurosurg. 52:80–86. 2017.
Article21. Matsuda R, Coello AF, De Benedictis A, Martinoni M, Duffau H. Awake mapping for resection of cavernous angioma and surrounding gliosis in the left dominant hemisphere: surgical technique and functional results: clinical article. J Neurosurg. 117:1076–1081. 2012.
Article22. Metting Z, Cerliani L, Rödiger LA, van der Naalt J. Pathophysiological concepts in mild traumatic brain injury: diffusion tensor imaging related to acute perfusion CT imaging. PLoS One. 8:e64461. 2013.
Article23. Min ZG, Niu C, Zhang QL, Zhang M, Qian YC. Optimal factors of diffusion tensor imaging predicting corticospinal tract injury in patients with brain tumors. Korean J Radiol. 18:844–851. 2017.
Article24. Mormina E, Longo M, Arrigo A, Alafaci C, Tomasello F, Calamuneri A, et al. MRI tractography of corticospinal tract and arcuate fasciculus in high-grade gliomas performed by constrained spherical deconvolution: qualitative and quantitative analysis. AJNR Am J Neuroradiol. 36:1853–1858. 2015.
Article25. Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 61:306–317. discussion 317-308. 2007.
Article26. Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 58(4 Suppl 2):ONS-292-ONS-303. discussion ONS-303-ONS-304. 2006.
Article27. Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 61:178–185. discussion 186. 2007.
Article28. Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices. 9:491–500. 2012.
Article29. Panigrahi M, Chandrasekhar YB, Vooturi S, Ram GA, Rammohan VS. Surgical resection of insular gliomas and roles of functional magnetic resonance imaging and diffusion tensor imaging tractography-single surgeon experience. World Neurosurg. 98:587–593. 2017.
Article30. Park ES, Cho YH, Kim JH, Kim SJ, Khang SK, Kim CJ. Frontal transcortical approach in 12 central neurocytomas. Acta Neurochir (Wien). 154:1961–1971. discussion 1972. 2012.
Article31. Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 201:637–648. 1996.
Article32. Potts MB, Chang EF, Young WL, Lawton MT; UCSF Brain AVM Study Project. Transsylvian-transinsular approaches to the insula and basal ganglia: operative techniques and results with vascular lesions. Neurosurgery. 70:824–834. discussion 834. 2012.33. Rosenstock T, Giampiccolo D, Schneider H, Runge SJ, Bährend I, Vajkoczy P, et al. Specific DTI seeding and diffusivity-analysis improve the quality and prognostic value of TMS-based deterministic DTI of the pyramidal tract. Neuroimage Clin. 16:276–285. 2017.
Article34. Shah PA. Transcranial motor evoked potential monitoring outcome in the high-risk brain and spine surgeries: correlation of clinical and neurophysiological data - an Indian perspective. Ann Indian Acad Neurol. 16:609–613. 2013.
Article35. Shahar T, Rozovski U, Marko NF, Tummala S, Ziu M, Weinberg JS, et al. Preoperative imaging to predict intraoperative changes in tumor-to-corticospinal tract distance: an analysis of 45 cases using high-field intraoperative magnetic resonance imaging. Neurosurgery. 75:23–30. 2014.
Article36. Shanmuganathan K, Zhuo J, Chen HH, Aarabi B, Adams J, Miller C, et al. Diffusion tensor imaging parameter obtained during acute blunt cervical spinal cord injury in predicting long-term outcome. J Neurotrauma. 34:2964–2971. 2017.
Article37. Sollmann N, Wildschuetz N, Kelm A, Conway N, Moser T, Bulubas L, et al. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J Neurosurg. 128:800–810. 2018.
Article38. Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 14:723–735. 2001.
Article39. Uribe JS, Vale FL. Limited access inferior temporal gyrus approach to mesial basal temporal lobe tumors. J Neurosurg. 110:137–146. 2009.
Article40. Vassal F, Schneider F, Sontheimer A, Lemaire JJ, Nuti C. Intraoperative visualisation of language fascicles by diffusion tensor imaging-based tractography in glioma surgery. Acta Neurochir (Wien). 155:437–448. 2013.
Article41. Weiss Lucas C, Tursunova I, Neuschmelting V, Nettekoven C, Oros-Peusquens AM, Stoffels G, et al. Functional MRI vs. navigated TMS to optimize M1 seed volume delineation for DTI tractography. A prospective study in patients with brain tumours adjacent to the corticospinal tract. Neuroimage Clin. 13:297–309. 2016.
Article42. Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 6:93–108. 2002.
Article43. Yu CS, Li KC, Xuan Y, Ji XM, Qin W. Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol. 56:197–204. 2005.
Article44. Zhang Y, Mao Z, Wei P, Jin Y, Ma L, Zhang J, et al. Preoperative prediction of location and shape of facial nerve in patients with large vestibular schwannomas using diffusion tensor imaging-based fiber tracking. World Neurosurg. 99:70–78. 2017.
Article45. Zhukov VY, Goryaynov SA, Ogurtsova AA, Ageev IS, Protskiy SV, Pronin IN, et al. Diffusion tensor imaging tractography and intraoperative neurophysiological monitoring in surgery of intracranial tumors located near the pyramidal tract. Zh Vopr Neirokhir Im N N Burdenko. 80:5–18. 2016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Tensor Imaging in Inflammatory and Neoplastic Intramedullary Spinal Cord Lesions: Focusing on Fiber Tracking
- Anisotropy Measurement and Fiber Tracking of the White Matter by Using Diffusion Tensor MR Imaging: Influence of the Number of Diffusion-Sensitizing Gradient Direction
- Clinical Use of Diffusion Tensor Image-Merged Functional Neuronavigation for Brain Tumor Surgeries: Review of Preoperative, Intraoperative, and Postoperative Data for 123 Cases
- Usefulness of DTI-based three dimensional corticospinal tractography in children with hemiplegic cerebral palsy
- Improving Delineation of the Corticospinal Tract in the Monkey Brain Scanned With Conventional Diffusion Tensor Imaging by Using a Compressed Sensing Based Algorithm