J Korean Neurosurg Soc.
2020 Mar;63(2):163-170. 10.3340/jkns.2019.0188.
Milk Fat Globule-Epidermal Growth Factor VIII Ameliorates Brain Injury in the Subacute Phase of Cerebral Ischemia in an Animal Model
- Affiliations
-
- 1Department of Neurosurgery, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 2Department of Neurosurgery, Anam Hospital, College of Medicine, Korea University, Seoul, Korea
- 3NEXEL Co., Ltd., Seoul, Korea
- 4Laboratory of Stem Cells and Tissue Regeneration, Department of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea
- 5Center of Innovative Cell Therapy and Research, Anam Hospital, College of Medicine, Korea University, Seoul, Korea
- KMID: 2501702
- DOI: http://doi.org/10.3340/jkns.2019.0188
Abstract
Objective
: Milk fat globule-epidermal growth factor VIII (MFG-E8) may play a key role in inflammatory responses and has the potential to function as a neuroprotective agent for ameliorating brain injury in cerebral infarction. This study aimed to determine the role of MFG-E8 in brain injury in the subacute phase of cerebral ischemia in a rat model.
Methods
: Focal cerebral ischemia was induced in rats by occluding the middle cerebral artery with the modified intraluminal filament technique. Twenty-four hours after ischemia induction, rats were randomly assigned to two groups and treated with either recombinant human MFG-E8 or saline. Functional outcomes were assessed using the modified Neurological Severity Score (mNSS), and infarct volumes were evaluated using histology. Anti-inflammation, angiogenesis, and neurogenesis were assessed using immunohistochemistry with antibodies against ionized calcium-binding adapter molecule 1 (Iba-1), rat endothelial cell antigen-1 (RECA-1), and bromodeoxyuridine (BrdU)/doublecortin (DCX), respectively.
Results
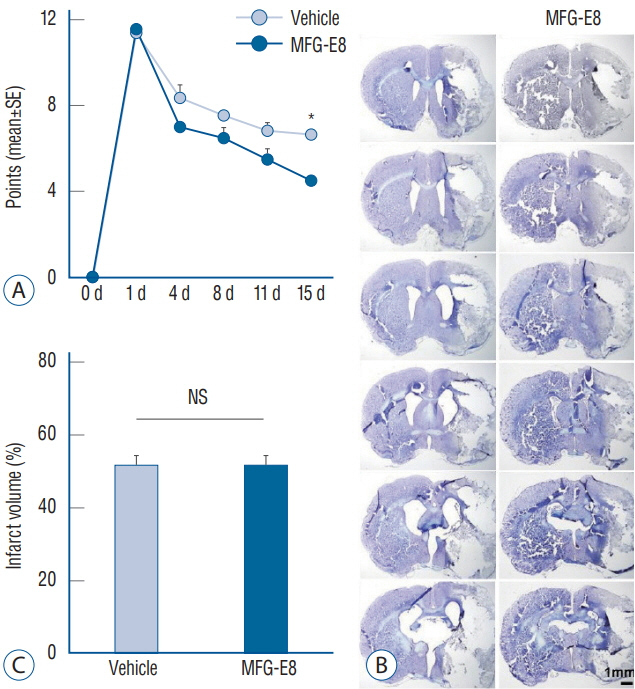
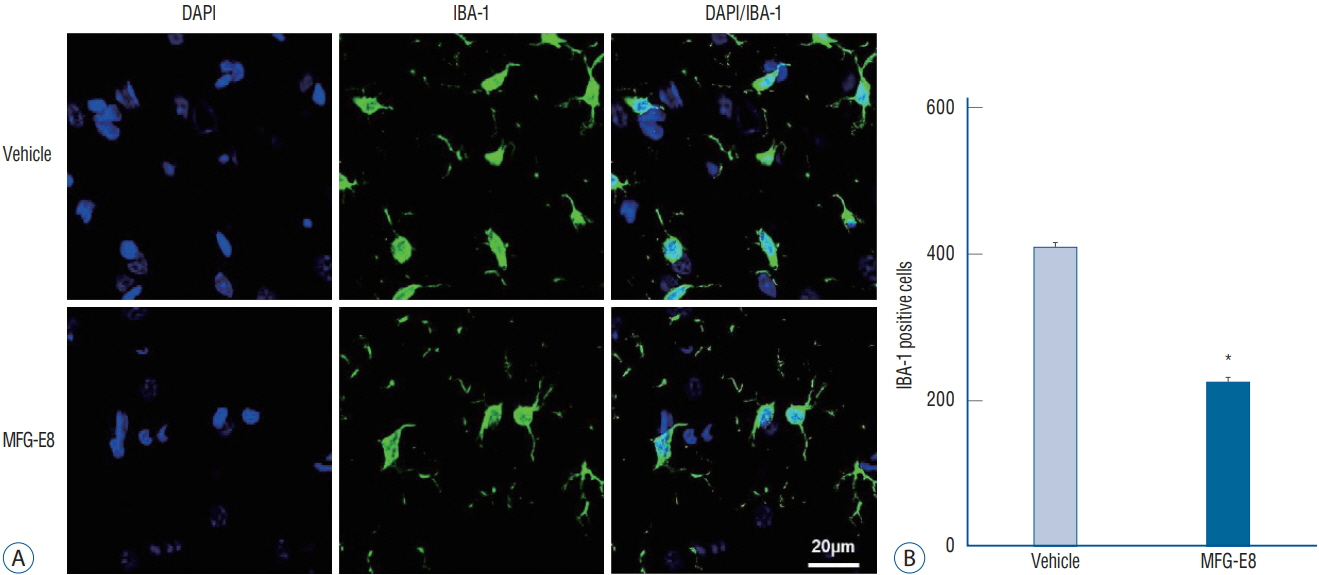
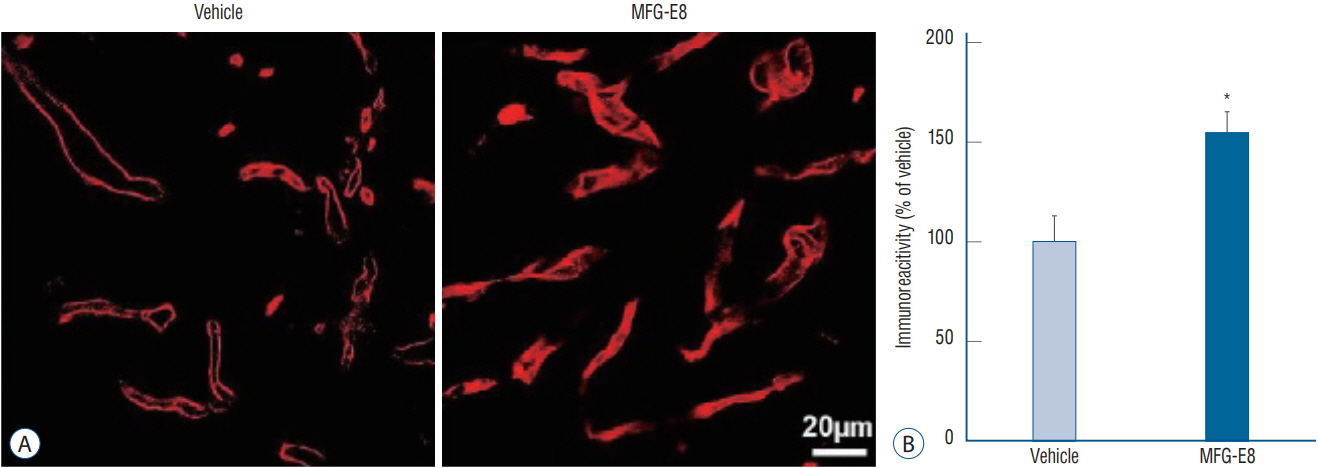
: Our results showed that intravenous MFG-E8 treatment did not reduce the infarct volume; however, the mNSS test revealed that neurobehavioral deficits were significantly improved in the MFG-E8-treated group than in the vehicle group. Immunofluorescence staining revealed a significantly lower number of Iba-1-positive cells and higher number of RECA-1 in the periinfarcted brain region, and significantly higher numbers of BrdU- and DCX-positive cells in the subventricular zone in the MFG-E8-treated group than in the vehicle group.
Conclusion
: Our findings suggest that MFG-E8 improves neurological function by suppressing inflammation and enhancing angiogenesis and neuronal proliferation in the subacute phase of cerebral infarction.
Keyword
Figure
Reference
-
References
1. Barone FC. Ischemic stroke intervention requires mixed cellular protection of the penumbra. Curr Opin Investig Drug. 10:220–223. 2009.2. Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 35:2385–2389. 2004.
Article3. Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339. 2009.
Article4. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 32:1005–1011. 2001.
Article5. Cheyuo C, Jacob A, Wu R, Zhou M, Qi L, Dong W, et al. Recombinant human MFG-E8 attenuates cerebral ischemic injury: its role in antiinflammation and anti-apoptosis. Neuropharmacology. 62:890–900. 2012.
Article6. Choi KS, Kim HJ, Do SH, Hwang SJ, Yi HJ. Neuroprotective effects of hydrogen inhalation in an experimental rat intracerebral hemorrhage model. Brain Res Bull. 142:122–128. 2018.
Article7. Choi TM, Yun M, Lee JK, Park JT, Park MS, Kim HS. Proteomic analysis of a rat cerebral ischemic injury model after human cerebral endothelial cell transplantation. J Korean Neurosurg Soc. 59:544–550. 2016.
Article8. Collins C, Nehlin JO, Stubbs JD, Kowbel D, Kuo WL, Parry G. Mapping of a newly discovered human gene homologous to the apoptosis associated-murine mammary protein, MFG-E8, to chromosome 15q25. Genomics. 39:117–118. 1997.
Article9. Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 22:1399–1419. 2002.
Article10. Fricker M, Neher JJ, Zhao JW, Théry C, Tolkovsky AM, Brown GC. MFGE8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci. 32:2657–2666. 2012.
Article11. Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 417:182–187. 2002.
Article12. Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 11:R795–R805. 2001.
Article13. Kim TG, Kwon O, Shin YS, Sung JH, Koh JS, Kim BT. Endovascular treatments performed collaboratively by the Society of Korean Endovascular Neurosurgeons Members : a nationwide multicenter survey. J Korean Neurosurg Soc. 62:502–518. 2019.
Article14. Lauber K, Keppeler H, Munoz LE, Koppe U, Schröder K, Yamaguchi H, et al. Milk fat globule-EGF factor 8 mediates the enhancement of apoptotic cell clearance by glucocorticoids. Cell Death Differ. 20:1230–1240. 2013.
Article15. Legos JJ, Whitmore RG, Erhardt JA, Parsons AA, Tuma RF, Barone FC. Quantitative changes in interleukin proteins following focal stroke in the rat. Neurosci Lett. 282:189–192. 2000.
Article16. Lu C, Hua F, Liu L, Ha T, Kalbfleisch J, Schweitzer J, et al. Scavenger receptor class-A has a central role in cerebral ischemia-reperfusion injury. J Cereb Blood Flow Metab. 30:1972–1981. 2010.
Article17. Miksa M, Amin D, Wu R, Jacob A, Zhou M, Dong W, et al. Maturationinduced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 22:743–748. 2008.
Article18. Miksa M, Amin D, Wu R, Ravikumar TS, Wang P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol Med. 13:553–560. 2007.
Article19. Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 25:586–593. 2006.
Article20. Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected]. J Immunol. 183:5983–5990. 2009.
Article21. Oshima K, Aoki N, Negi M, Kishi M, Kitajima K, Matsuda T. Lactationdependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem Biophys Res Commun. 254:522–528. 1999.
Article22. Phan TG, Wright PM, Markus R, Howells DW, Davis SM, Donnan GA. Salvaging the ischaemic penumbra: more than just reperfusion? Clin Exp Pharmacol Physiol. 29:1–10. 2002.
Article23. Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2:965–975. 2002.
Article24. Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 183:25–33. 2003.
Article25. Silvestre JS, Théry C, Hamard G, Boddaert J, Aguilar B, Delcayre A, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 11:499–506. 2005.
Article26. Stubbs JD, Lekutis C, Singer KL, Bui A, Yuzuki D, Srinivasan U, et al. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc Natl Acad Sci U S A. 87:8417–8421. 1990.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Animal Model of Cerebral Ischemia
- Epidermal growth factor receptors increase in rabbit embryonal implantation
- Amplification of epidermal growth factor receptor gene in primary cervical cancer
- Amplification of epidermal growth factor receptor gene in primary cervical cancer
- Change of Water Content in Rat Brain During the Expertmental Development of Cerebral Ischemia