Validation of prognostic impact of ADV score for resection of hepatocellular carcinoma: analysis using Korea Liver Cancer Registry Database
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Surgery, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2500824
- DOI: http://doi.org/10.4174/astr.2020.98.5.235
Abstract
- Purpose
We aimed to validate the prognostic predictive power of ADV score (α-FP-des-γ-carboxyprothrombin [DCP]-tumor volume [TV] score, calculated as α-FP [ng/mL] × DCP [mAU/mL] × TV [mL] and expressed in log10) for predicting patient survival after resection of hepatocellular carcinoma (HCC).
Methods
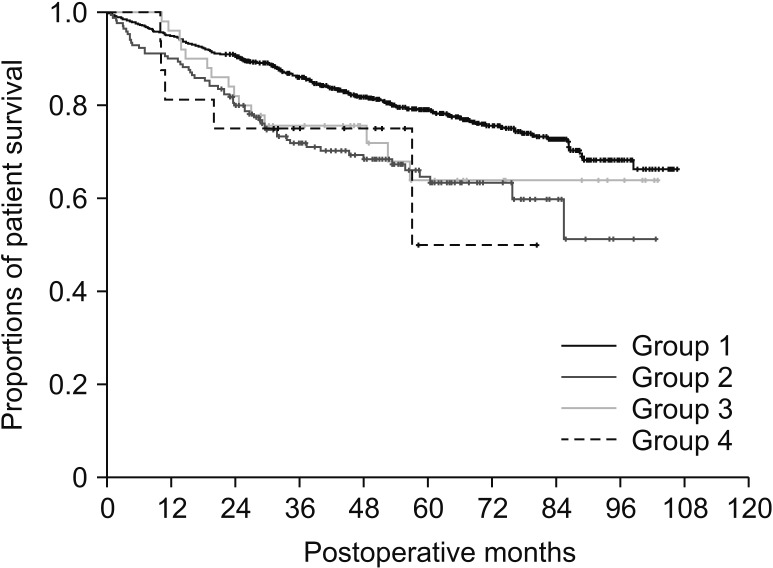
This study included 1,390 patients with HCC registered in the Korea Liver Cancer Registry. Patients underwent hepatic resection between 2008 and 2012 and were followed up until December 2016. They were divided into 4 groups according to the number of tumors and preoperative treatment.
Results
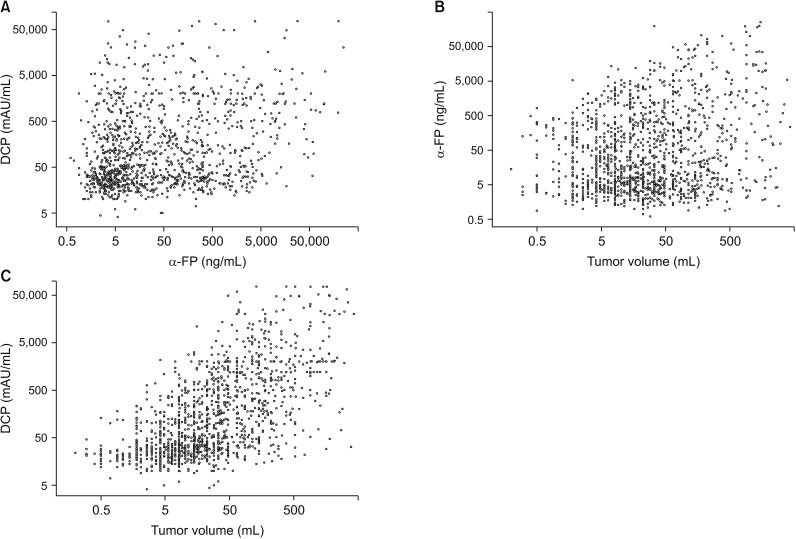
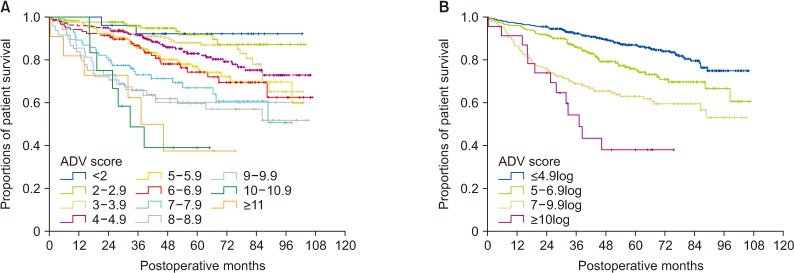
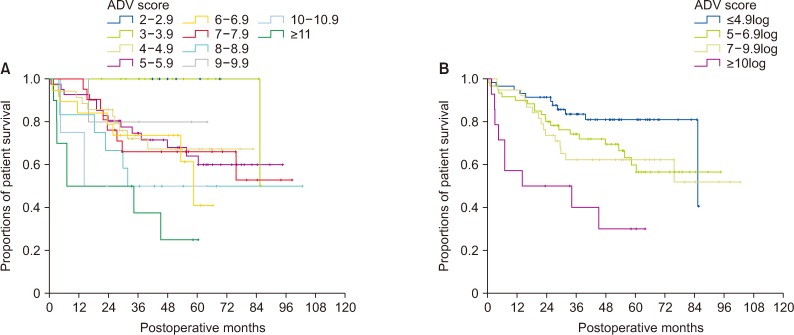
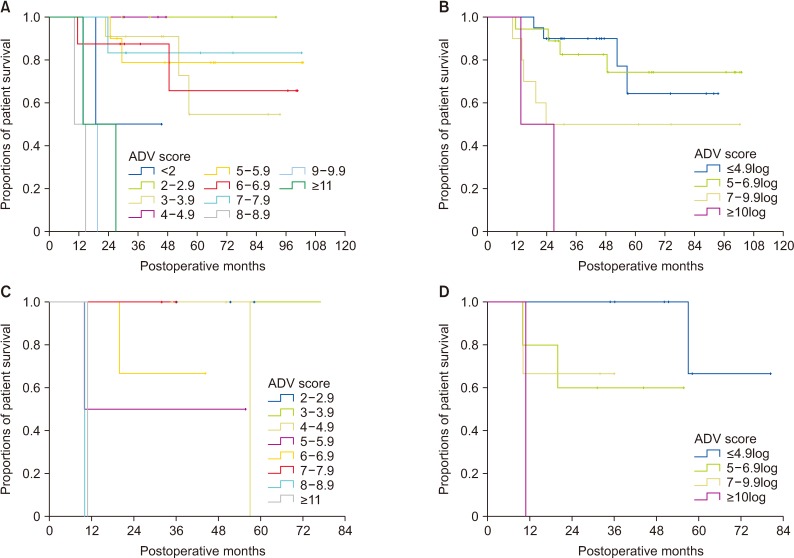
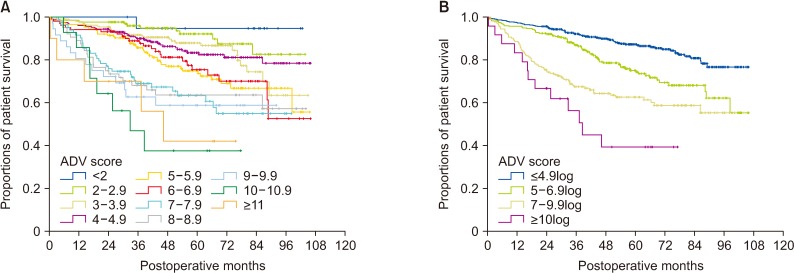
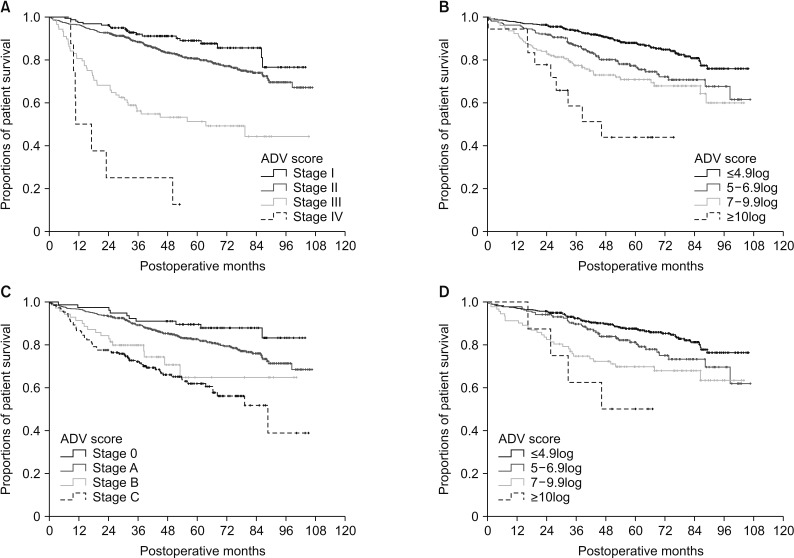
There was no significant correlation among α-FP, DCP, and TV values (r2 ≤ 0.04, P < 0.001). In group 1 with single treatment-naive tumor (n = 1,154), patient stratification with postoperative ADV 1log-interval and cutoffs of 5log, 7log, and 10log showed great prognostic contrast (P < 0.001). In group 2 with multiple treatment-naive tumors (n = 170), patient stratification with postoperative ADV 1log-interval and above-mentioned 3 cutoffs also showed great prognostic contrast (P < 0.001). In group 3 (n = 50) and group 4 (n = 16) with preoperative-treated tumors, patient stratification with postoperative ADV 1log-interval and above-mentioned 3 cutoffs showed noticeable prognostic contrast (P ≤ 0.031). Preoperative ADV score based on preoperative findings also showed great prognostic contrast in 1,106 patients preoperatively diagnosed as having single treatment-naive tumor (P < 0.001). Confining patients to tumor-node-metastasis stages I and II (n = 1,072) as well as Barcelona Clinic Liver Cancer stage 0 and A (n = 862), postoperative ADV cutoffs showed further prognostic stratification.
Conclusion
This validation study strongly suggests that ADV score is an integrated surrogate marker for postresection prognosis in patients with HCC.
Figure
Cited by 3 articles
-
Prognostic impact of serum soluble PD-1 and ADV score for living donor liver transplantation in patients with previously untreated hepatocellular carcinoma
Shin Hwang, Kyung Jin Lee, Deok-Bog Moon, Gi-Won Song, Dong-Hwan Jung, Yun Kyu Kim, Hunji Yang, Da Eun An, Sion Lee, Sung-Gyu Lee
Ann Surg Treat Res. 2022;102(1):46-54. doi: 10.4174/astr.2022.102.1.46.Evolving trends in treatment patterns for hepatocellular carcinoma in Korea from 2008 to 2022: a nationwide population-based study
Ji Won Han, Won Sohn, Gwang Hyeon Choi, Jeong Won Jang, Gi Hyeon Seo, Bo Hyun Kim, Jong Young Choi
J Liver Cancer. 2024;24(2):274-285. doi: 10.17998/jlc.2024.08.13.ADV score is a reliable surrogate biomarker of hepatocellular carcinoma in liver resection and transplantation
Shin Hwang, Dong-Hwan Jung, Gi-Won Song
Ann Liver Transplant. 2023;3(2):86-93. doi: 10.52604/alt.23.0023.
Reference
-
1. Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015; 19:1281–1290. PMID: 25956724.
Article2. Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Multiplication of tumor volume by two tumor markers is a post-resection prognostic predictor for solitary hepatocellular carcinoma. J Gastrointest Surg. 2016; 20:1807–1820. PMID: 27311982.
Article3. Jung DH, Hwang S, Lee YJ, Kim KH, Song GW, Ahn CS, et al. Small hepatocellular carcinoma with low tumor marker expression benefits more from anatomical resection than tumors with aggressive biology. Ann Surg. 2019; 269:511–519. PMID: 28837444.
Article4. Hwang S, Joh JW, Wang HJ, Kim DG, Kim KS, Suh KS, et al. Prognostic prediction models for resection of large hepatocellular carcinoma: a Korean multicenter study. World J Surg. 2018; 42:2579–2591. PMID: 29340726.
Article5. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016; 22:18–75. PMID: 27044762.6. Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford). 2005; 7:35–41. PMID: 18333159.
Article7. Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, et al. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010; 116:3006–3014. PMID: 20564406.8. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocel lular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003; 38:207–215. PMID: 12673442.9. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer;2010.10. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID: 21374666.
Article11. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014; 146:1691–1700. PMID: 24583061.
Article12. Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009; 29:502–510. PMID: 19141028.
Article13. Zhang H, Yuan SX, Dai SY, Zhang JM, Huang X, Lu CD, et al. Tumor size does not independently affect long-term survival after curative resection of solitary hepatocellular carcinoma without macroscopic vascular invasion. World J Surg. 2014; 38:947–957. PMID: 24258262.
Article14. Meguro M, Mizuguchi T, Nishidate T, Okita K, Ishii M, Ota S, et al. Prognostic roles of preoperative α-fetoprotein and des- γ-carboxy prothrombin in hepatocellular carcinoma patients. World J Gastroenterol. 2015; 21:4933–4945. PMID: 25945007.15. Kiriyama S, Uchiyama K, Ueno M, Ozawa S, Hayami S, Tani M, et al. Triple positive tumor markers for hepatocellular carcinoma are useful predictors of poor survival. Ann Surg. 2011; 254:984–991. PMID: 21606837.
Article16. Nakagawa S, Beppu T, Okabe H, Sakamoto K, Kuroki H, Mima K, et al. Triple positive tumor markers predict recurrence and survival in early stage hepatocellular carcinoma. Hepatol Res. 2014; 44:964–974. PMID: 24245496.
Article17. Suh SW, Lee KW, Lee JM, You T, Choi Y, Kim H, et al. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J Hepatol. 2014; 60:1219–1224. PMID: 24548529.
Article18. Kamiyama T, Yokoo H, Kakisaka T, Orimo T, Wakayama K, Kamachi H, et al. Multiplication of alpha-fetoprotein and protein induced by vitamin K absence-II is a powerful predictor of prognosis and recurrence in hepatocellular carcinoma patients after a hepatectomy. Hepatol Res. 2015; 45:E21–E31. PMID: 25382703.
Article19. Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, et al. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology. 2016; 279:432–442. PMID: 26653683.
Article20. Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, et al. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015; 40:843–851. PMID: 25253426.
Article21. Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol. 2014; 203:W253–W259. PMID: 25148181.
Article22. Min JH, Kim YK, Lim S, Jeong WK, Choi D, Lee WJ. Prediction of microvascular invasion of hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging: impact of intra-tumoral fat detected on chemical-shift images. Eur J Radiol. 2015; 84:1036–1043. PMID: 25818729.
Article23. Shirabe K, Toshima T, Kimura K, Yamashita Y, Ikeda T, Ikegami T, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int. 2014; 34:937–941. PMID: 24393295.
Article24. Pote N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015; 62:848–854. PMID: 25450201.25. Ha SM, Hwang S, Park JY, Lee YJ, Kim KH, Song GW, et al. Validation of the OncoHepa test, a multigene expression profile test, and the tumor marker-volume score to predict postresection outcome in small solitary hepatocellular carcinomas. Ann Surg Treat Res. 2018; 95:303–311. PMID: 30505821.26. Kang WH, Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, et al. Prognostic effect of transarterial chemoembolization-induced complete pathological response in patients undergoing liver resection and transplantation for hepatocellular carcinoma. Liver Transpl. 2017; 23:781–790. PMID: 28240808.27. Ha TY, Hwang S, Lee YJ, Kim KH, Ko GY, Gwon DI, et al. Absence of benefit of transcatheter arterial chemoembolization (TACE) in patients with resectable solitary hepatocellular carcinoma. World J Surg. 2016; 40:1200–1210. PMID: 26666422.28. Xu W, Kwon JH, Moon YH, Kim YB, Yu YS, Lee N, et al. Influence of preoperative transcatheter arterial chemoembolization on gene expression in the HIF-1α pathway in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014; 140:1507–1515. PMID: 24853275.29. Galizia MS, Tore HG, Chalian H, Yaghmai V. Evaluation of hepatocellular carcinoma size using two-dimensional and volumetric analysis: effect on liver transplantation eligibility. Acad Radiol. 2011; 18:1555–1560. PMID: 21962475.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ADV score is a reliable surrogate biomarker of hepatocellular carcinoma in liver resection and transplantation
- Construction and validation of a preoperative prognostic model integrating the novel aspartate aminotransferasealbumin score for hepatocellular carcinoma patients undergoing liver resection
- Prediction of Post-resection Prognosis Using the ADV Score for Huge Hepatocellular Carcinomas ≥13 cm
- Analysis of posttransplant hepatocellular carcinoma prognosis using ADV score: a validation multicenter study
- Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis