Int J Stem Cells.
2020 Mar;13(1):142-150. 10.15283/ijsc19073.
IL6 Receptor Facilitates Adipogenesis Differentiation of Human Mesenchymal Stem Cells through Activating P38 Pathway
- Affiliations
-
- 1Center for Biotherapy, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 2Organ Transplant Center, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 3Department of Orthopedics, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- KMID: 2500573
- DOI: http://doi.org/10.15283/ijsc19073
Abstract
- Background and Objectives
Mesenchymal stem cells (MSCs) have the multipotent capacity to differentiate into multiple tissue lineages as well as to self-renew, which is the main origin of adipocytes. IL6/IL6R pathway exerts a significant role in tissue regeneration and cell differentiation. Whereas, the underlying mechanism between IL6/IL6R pathway and MSCs adipogenesis differentiation remains elusive.
Methods
MSCs from healthy donors were cultured in adipogenesis differentiation medium for 0∼14 days, during which their adipogenesis differentiation degree was evaluated by Oil Red O staining. The expression of IL6R was detected in MSCs during adipogenesis differentiation. Knockdown and overexpression of IL6R were respectively performed using siRNA and lentivirus to investigate its effect on MSCs adipogenesis differentiation. The adipogenesis marker genes expression and MAPK pathway activation were detected by Western blotting. The role of P38 pathway in the adipogenesis differentiation of MSCs was determined using the specific inhibitor SB203580.
Results
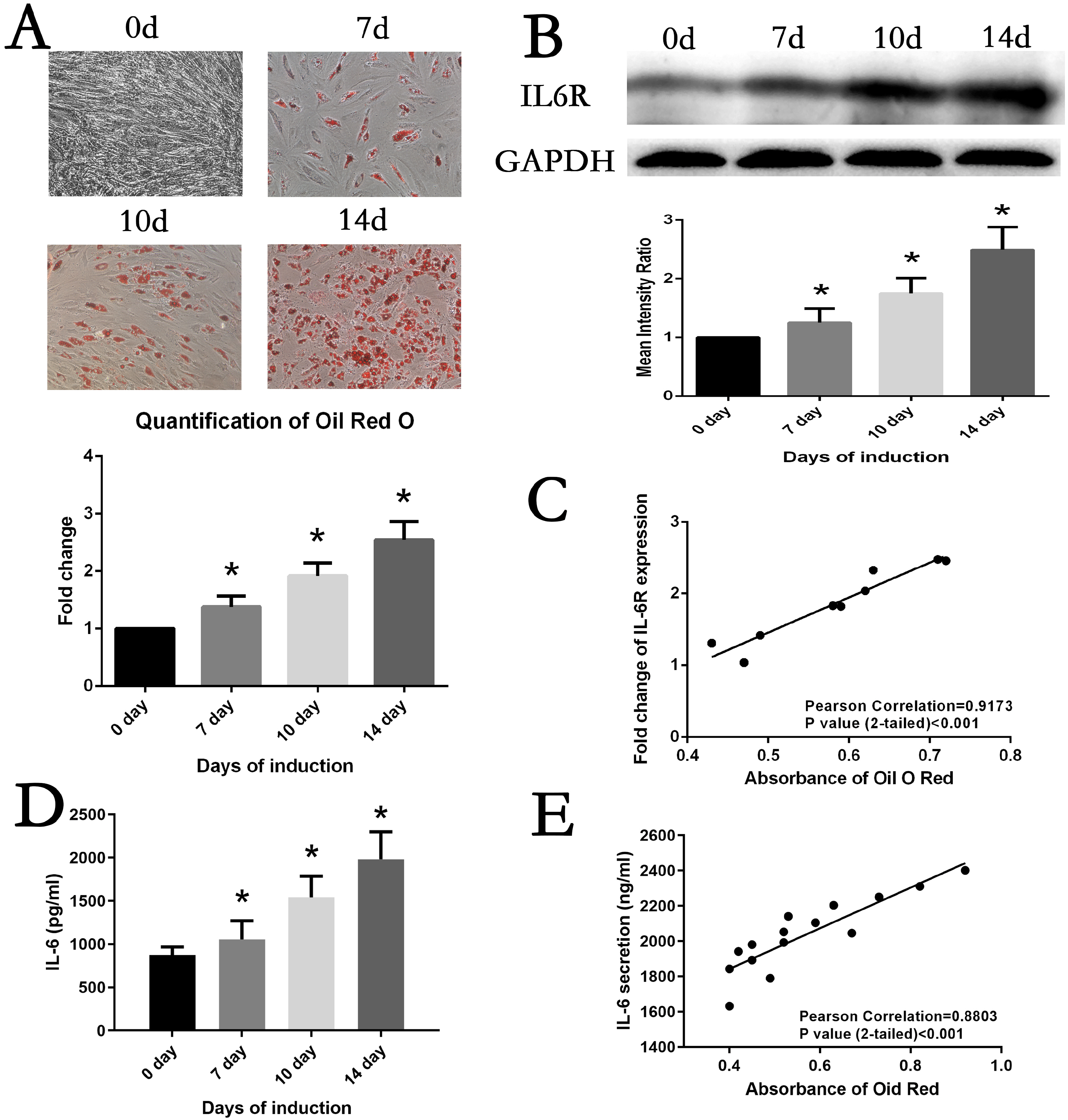
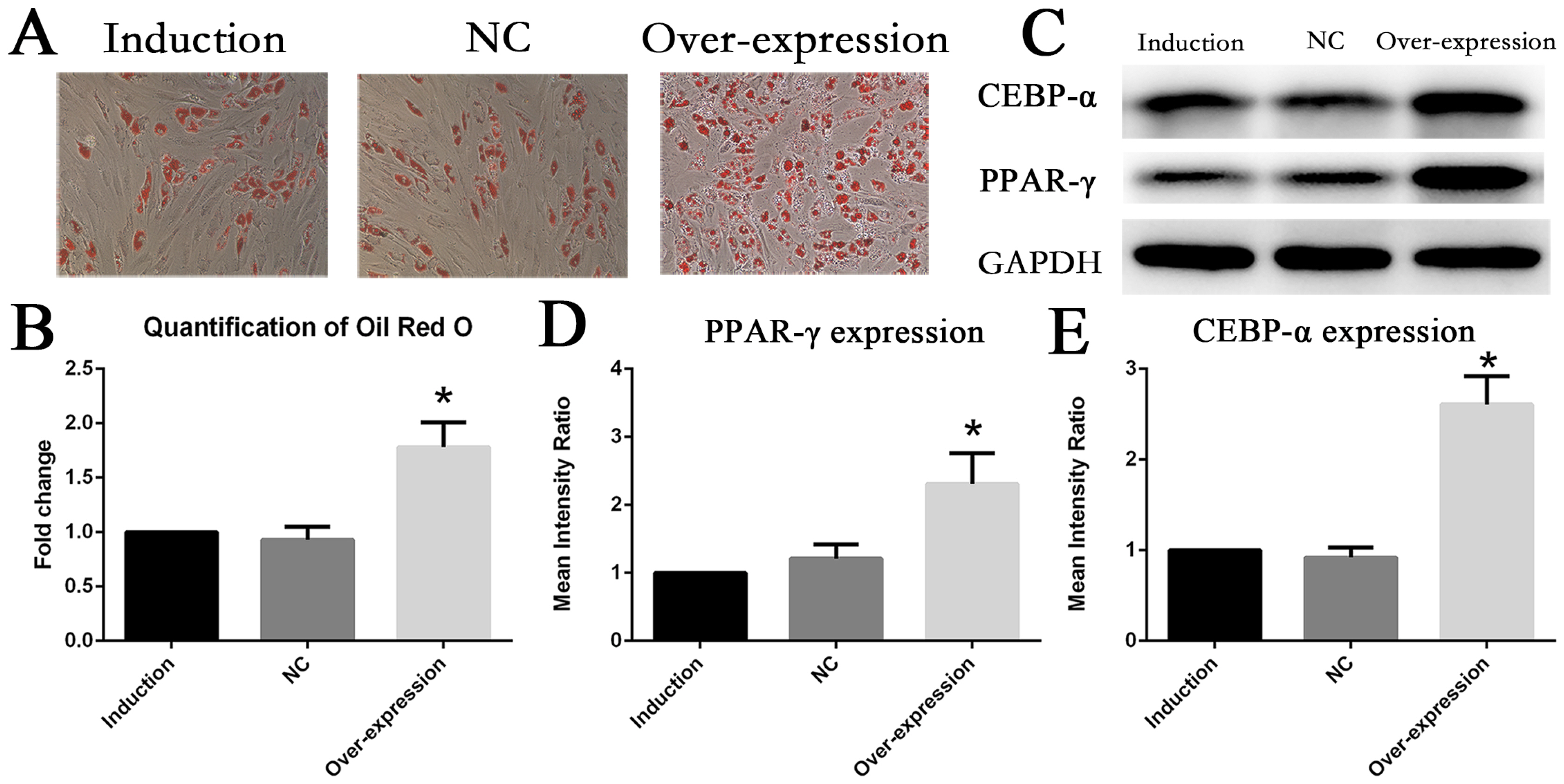
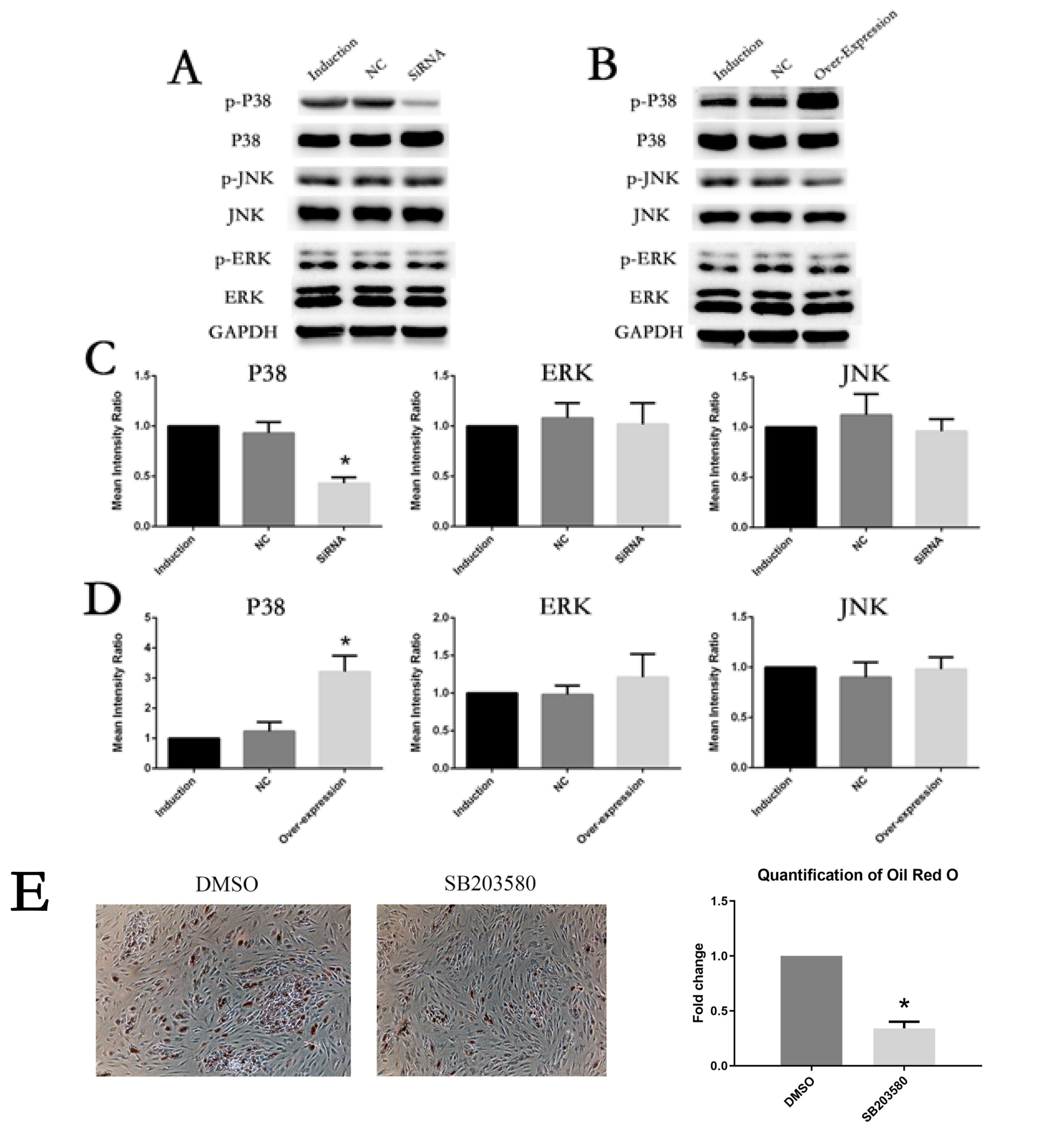
The expression of IL6 and IL6R increased during adipogenesis differentiation in MSCs, which were positively correlated with Oil Red O quantification result. Knockdown and overexpression experiments demonstrated a positive correlation between the expressions of IL6R and MSCs adipogenesis differentiation, accompanied by same trend of P38 phosphorylation. Besides, the specific P38 inhibitor SB203580 markedly inhibited the adipogenesis differentiation potential of MSCs.
Conclusions
This study reveals IL6R facilitates the adiogenesis differentiation of MSCs via activating P38 pathway.
Keyword
Figure
Reference
-
References
1. Jackson WM, Nesti LJ, Tuan RS. 2012; Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med. 1:44–50. DOI: 10.5966/sctm.2011-0024. PMID: 23197639. PMCID: PMC3727688.
Article2. Matsushita K, Dzau VJ. 2017; Mesenchymal stem cells in obesity: insights for translational applications. Lab Invest. 97:1158–1166. DOI: 10.1038/labinvest.2017.42. PMID: 28414326.
Article3. Lee CW, Hsiao WT, Lee OK. 2017; Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Transl Res. 182:61–74.e8. DOI: 10.1016/j.trsl.2016.11.003. PMID: 27908750.
Article4. Hunter CA, Jones SA. 2015; IL-6 as a keystone cytokine in health and disease. Nat Immunol. 16:448–457. DOI: 10.1038/ni.3153. PMID: 25898198.
Article5. Ouchi N, Parker JL, Lugus JJ, Walsh K. 2011; Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 11:85–97. DOI: 10.1038/nri2921. PMID: 21252989. PMCID: PMC3518031.
Article6. Pickup JC, Mattock MB, Chusney GD, Burt D. 1997; NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 40:1286–1292. DOI: 10.1007/s001250050822. PMID: 9389420.
Article7. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. 2001; C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 286:327–334. DOI: 10.1001/jama.286.3.327. PMID: 11466099.
Article8. Theurich S, Tsaousidou E, Hanssen R, Lempradl AM, Mauer J, Timper K, Schilbach K, Folz-Donahue K, Heilinger C, Sexl V, Pospisilik JA, Wunderlich FT, Brüning JC. 2017; IL-6/Stat3-Dependent induction of a distinct, obesity-associated NK cell subpopulation deteriorates energy and glucose homeostasis. Cell Metab. 26:171–184.e6. DOI: 10.1016/j.cmet.2017.05.018. PMID: 28683285.
Article9. Ng J, Hynes K, White G, Sivanathan KN, Vandyke K, Bartold PM, Gronthos S. 2016; Immunomodulatory properties of induced pluripotent stem cell‐derived mesenchymal cells. J Cell Biochem. 117:2844–2853. DOI: 10.1002/jcb.25596. PMID: 27167148.
Article10. Xie Z, Tang S, Ye G, Wang P, Li J, Liu W, Li M, Wang S, Wu X, Cen S, Zheng G, Ma M, Wu Y, Shen H. 2018; Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 9:13. DOI: 10.1186/s13287-017-0766-0. PMID: 29357923. PMCID: PMC5776773.
Article11. Kondo M, Yamaoka K, Sakata K, Sonomoto K, Lin L, Nakano K, Tanaka Y. 2015; Contribution of the Interleukin-6/STAT-3 signaling pathway to chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheumatol. 67:1250–1260. DOI: 10.1002/art.39036. PMID: 25604648.
Article12. James AW. 2013; Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica. 2013:684736. DOI: 10.1155/2013/684736. PMID: 24416618. PMCID: PMC3874981.
Article13. Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, Li D, Wu Y, Shen H. 2016; Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 68:430–440. DOI: 10.1002/art.39433. PMID: 26413886.
Article14. Farmer SR. 2006; Transcriptional control of adipocyte formation. Cell Metab. 4:263–273. DOI: 10.1016/j.cmet.2006.07.001. PMID: 17011499. PMCID: PMC1958996.
Article15. Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishimoto T. 1988; Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 241:825–828. DOI: 10.1126/science.3136546. PMID: 3136546.
Article16. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999; Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.
Article17. Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. 1999; The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 265:134–139. DOI: 10.1006/bbrc.1999.1620. PMID: 10548503.
Article18. Kyriakis JM, Avruch J. 2012; Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 92:689–737. DOI: 10.1152/physrev.00028.2011. PMID: 22535895.
Article19. Legendre F, Bogdanowicz P, Boumediene K, Pujol JP. 2005; Role of interleukin 6 (IL-6)/IL-6R-induced signal tranducers and activators of transcription and mitogen-activated protein kinase/extracellular. J Rheumatol. 32:1307–1316. PMID: 15996070.20. Chen JD, Xu FF, Zhu H, Li XM, Tang B, Liu YL, Zhang Y. 2014; [ICAM-1 regulates differentiation of MSC to adipocytes via activating MAPK pathway]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 22:160–165. DOI: 10.7534/j.issn.1009-2137.2014.01.031. PMID: 24598670. Chinese.21. Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. 2009; Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 108:577–588. DOI: 10.1002/jcb.22289. PMID: 19650110. PMCID: PMC2774119.
Article22. Aouadi M, Jager J, Laurent K, Gonzalez T, Cormont M, Binétruy B, Le Marchand-Brustel Y, Tanti JF, Bost F. 2007; p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett. 581:5591–5596. DOI: 10.1016/j.febslet.2007.10.064. PMID: 17997987.
Article23. Aouadi M, Laurent K, Prot M, Le Marchand-Brustel Y, Binétruy B, Bost F. 2006; Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes. 55:281–289. DOI: 10.2337/diabetes.55.02.06.db05-0963. PMID: 16443758.
Article24. Batchvarova N, Wang XZ, Ron D. 1995; Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153). EMBO J. 14:4654–4661. DOI: 10.1002/j.1460-2075.1995.tb00147.x. PMID: 7588595. PMCID: PMC394562.
Article25. Sonowal H, Kumar A, Bhattacharyya J, Gogoi PK, Jaganathan BG. 2013; Inhibition of actin polymerization decreases osteogeneic differentiation of mesenchymal stem cells through p38 MAPK pathway. J Biomed Sci. 20:71. DOI: 10.1186/1423-0127-20-71. PMID: 24070328. PMCID: PMC3849435.
Article26. Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. 1994; Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord. 18:79–83. PMID: 8148928.27. Prior BM, Cureton KJ, Modlesky CM, Evans EM, Sloniger MA, Saunders M, Lewis RD. 1997; In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J Appl Physiol (1985). 83:623–630. DOI: 10.1152/jappl.1997.83.2.623. PMID: 9262461.
Article28. Matsushita K. 2016; Mesenchymal stem cells and metabolic syndrome: current understanding and potential clinical implications. Stem Cells Int. 2016:2892840. DOI: 10.1155/2016/2892840. PMID: 27313625. PMCID: PMC4903149.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carnosol induces the osteogenic differentiation of bone marrowderived mesenchymal stem cells via activating BMP-signaling pathway
- Regulation of Adipocyte Differentiation via MicroRNAs
- Synergistic Effect of Hydrogen and 5-Aza on Myogenic Differentiation through the p38 MAPK Signaling Pathway in Adipose-Derived Mesenchymal Stem Cells
- Primary Cilia Mediate Wnt5a/β-catenin Signaling to Regulate Adipogenic Differentiation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Following Calcium Induction
- The N- and C-terminal domains of parathyroid hormone-related protein affect differently the osteogenic and adipogenic potential of human mesenchymal stem cells