Int J Stem Cells.
2020 Mar;13(1):80-92. 10.15283/ijsc19097.
Robust and Reproducible Generation of Induced Neural Stem Cells from Human Somatic Cells by Defined Factors
- Affiliations
-
- 1Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea
- 2Department of Neuroscience, School of Medicine and Center for Neuroscience Research, Konkuk University, Seoul, Korea
- 3School of Cell and Molecular Medicine, University of Bristol, Bristol, UK
- 4Department of Stem Cell & Regenerative Biotechnology, Konkuk University, Seoul, Korea
- 5Department of Cell and Developmental Biology, Max Planck Institute for Molecular Biomedicine, Münster, Germany
- 6Department of Animal Sciences, Chungbuk National University, Cheongju, Korea
- 7School of Biotechnology and Healthcare, Wuyi University, Jiangmen, China
- KMID: 2500568
- DOI: http://doi.org/10.15283/ijsc19097
Abstract
- Background and Objectives
Recent studies have described direct reprogramming of mouse and human somatic cells into induced neural stem cells (iNSCs) using various combinations of transcription factors. Although iNSC technology holds a great potential for clinical applications, the low conversion efficiency and limited reproducibility of iNSC generation hinder its further translation into the clinic, strongly suggesting the necessity of highly reproducible method for human iNSCs (hiNSCs). Thus, in orderto develop a highly efficient and reproducible protocol for hiNSC generation, we revisited the reprogramming potentials of previously reported hiNSC reprogramming cocktails by comparing the reprogramming efficiency of distinct factor combinations including ours.
Methods
We introduced distinct factor combinations, OSKM (OCT4+SOX2+KLF4+C-MYC), OCT4 alone, SOX2 alone, SOX2+HMGA2, BRN4+SKM+SV40LT (BSKMLT), SKLT, SMLT, and SKMLT and performed comparative analysis of reprogramming potentials of distinct factor combinations in hiNSC generation.
Results
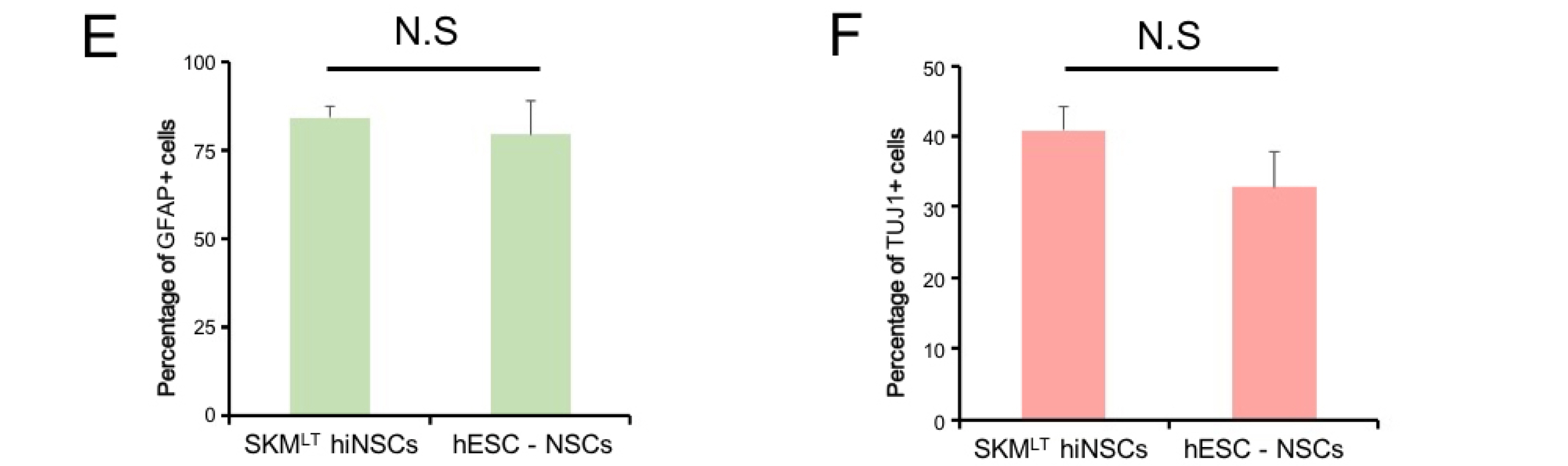
Here we show that ectopic expression of five reprogramming factors, BSKMLT leads the robust hiNSC generation (>80 folds enhanced efficiency) from human somatic cells compared with previously described factor combinations. With our combination, we were able to observe hiNSC conversion within 7 days of transduction. Throughout further optimization steps, we found that both BRN4 and KLF4 are not essential for hiNSC conversion.
Conclusions
Our factor combination could robustly and reproducibly generate hiNSCs from human somatic cells with distinct origins. Therefore, our novel reprogramming strategy might serve as a useful tool for hiNSC-based clinical application.
Keyword
Figure
Reference
-
References
1. Gage FH. 2000; Mammalian neural stem cells. Science. 287:1433–1438. DOI: 10.1126/science.287.5457.1433. PMID: 10688783.
Article2. Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. 2009; Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 106:13594–13599. DOI: 10.1073/pnas.0901402106. PMID: 19633196. PMCID: PMC2715325.
Article3. Martino G, Pluchino S. 2006; The therapeutic potential of neural stem cells. Nat Rev Neurosci. 7:395–406. DOI: 10.1038/nrn1908. PMID: 16760919.
Article4. Xuan AG, Luo M, Ji WD, Long DH. 2009; Effects of engrafted neural stem cells in Alzheimer's disease rats. Neurosci Lett. 450:167–171. DOI: 10.1016/j.neulet.2008.12.001. PMID: 19070649.
Article5. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article6. Lim KT, Kim J, Hwang SI, Zhang L, Han H, Bae D, Kim KP, Hu YP, Schöler HR, Lee I, Hui L, Han DW. 2018; Direct conversion of mouse fibroblasts into cholangiocyte progenitor cells. Stem Cell Reports. 10:1522–1536. DOI: 10.1016/j.stemcr.2018.03.002. PMID: 29606616. PMCID: PMC5995161.
Article7. Kim JH, Kim KP, Lim KT, Lee SC, Yoon JY, Song GQ, Hwang SI, Schöler HR, Cantz T, Han DW. 2015; Generation of integration-free induced hepatocyte-like cells from mouse fibroblasts. Sci Rep. 5:15706. DOI: 10.1038/srep15706. PMID: 26503743. PMCID: PMC4621602.
Article8. Kim JB, Lee H, Araúzo-Bravo MJ, Hwang K, Nam D, Park MR, Zaehres H, Park KI, Lee SJ. 2015; Oct4-induced oligodendrocyte progenitor cells enhance functional recovery in spinal cord injury model. EMBO J. 34:2971–2983. DOI: 10.15252/embj.201592652. PMID: 26497893. PMCID: PMC4687687.
Article9. Han DW, Greber B, Wu G, Tapia N, Araúzo-Bravo MJ, Ko K, Bernemann C, Stehling M, Schöler HR. 2011; Direct reprogramming of fibroblasts into epiblast stem cells. Nat Cell Biol. 13:66–71. DOI: 10.1038/ncb2136. PMID: 21131959.
Article10. Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Schöler HR. 2012; Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 10:465–472. DOI: 10.1016/j.stem.2012.02.021. PMID: 22445517.
Article11. Kim SM, Flaβkamp H, Hermann A, Araúzo-Bravo MJ, Lee SC, Lee SH, Seo EH, Lee SH, Storch A, Lee HT, Schöler HR, Tapia N, Han DW. 2014; Direct conversion of mouse fibroblasts into induced neural stem cells. Nat Protoc. 9:871–881. DOI: 10.1038/nprot.2014.056. PMID: 24651499.
Article12. Lim KT, Lee SC, Gao Y, Kim KP, Song G, An SY, Adachi K, Jang YJ, Kim J, Oh KJ, Kwak TH, Hwang SI, You JS, Ko K, Koo SH, Sharma AD, Kim JH, Hui L, Cantz T, Schöler HR, Han DW. 2016; Small molecules facilitate single factor-mediated hepatic reprogramming. Cell Rep. 15:814–829. DOI: 10.1016/j.celrep.2016.03.071. PMID: 27149847.
Article13. Hemmer K, Zhang M, van Wüllen T, Sakalem M, Tapia N, Baumuratov A, Kaltschmidt C, Kaltschmidt B, Schöler HR, Zhang W, Schwamborn JC. 2014; Induced neural stem cells achieve long-term survival and functional integration in the adult mouse brain. Stem Cell Reports. 3:423–431. DOI: 10.1016/j.stemcr.2014.06.017. PMID: 25241741. PMCID: PMC4265999.
Article14. Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, Tapia N, Lim KT, Kim J, Ahn HS, Ko K, Shin CY, Lee HT, Schöler HR, Hyun JK, Han DW. 2014; Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 289:32512–32525. DOI: 10.1074/jbc.M114.588871. PMID: 25294882. PMCID: PMC4239606.
Article15. Lu J, Liu H, Huang CT, Chen H, Du Z, Liu Y, Sherafat MA, Zhang SC. 2013; Generation of integration-free and region-specific neural progenitors from primate fibroblasts. Cell Rep. 3:1580–1591. DOI: 10.1016/j.celrep.2013.04.004. PMID: 23643533. PMCID: PMC3786191.
Article16. Maza I, Caspi I, Zviran A, Chomsky E, Rais Y, Viukov S, Geula S, Buenrostro JD, Weinberger L, Krupalnik V, Hanna S, Zerbib M, Dutton JR, Greenleaf WJ, Massarwa R, Novershtern N, Hanna JH. 2015; Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat Biotechnol. 33:769–774. DOI: 10.1038/nbt.3270. PMID: 26098448. PMCID: PMC4500825.
Article17. Bar-Nur O, Verheul C, Sommer AG, Brumbaugh J, Schwarz BA, Lipchina I, Huebner AJ, Mostoslavsky G, Hochedlinger K. 2015; Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage. Nat Biotechnol. 33:761–768. DOI: 10.1038/nbt.3247. PMID: 26098450. PMCID: PMC4840929.
Article18. Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. 2012; Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 11:100–109. DOI: 10.1016/j.stem.2012.05.018. PMID: 22683203. PMCID: PMC3399516.
Article19. Zhu S, Ambasudhan R, Sun W, Kim HJ, Talantova M, Wang X, Zhang M, Zhang Y, Laurent T, Parker J, Kim HS, Zaremba JD, Saleem S, Sanz-Blasco S, Masliah E, McKercher SR, Cho YS, Lipton SA, Kim J, Ding S. 2014; Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 24:126–129. DOI: 10.1038/cr.2013.156. PMID: 24296783. PMCID: PMC3879704.
Article20. Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, Zanetta C, Faravelli I, Bresolin N, Comi GP. 2012; Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res. 318:1528–1541. DOI: 10.1016/j.yexcr.2012.02.040. PMID: 22426197. PMCID: PMC3405531.
Article21. Yu KR, Shin JH, Kim JJ, Koog MG, Lee JY, Choi SW, Kim HS, Seo Y, Lee S, Shin TH, Jee MK, Kim DW, Jung SJ, Shin S, Han DW, Kang KS. 2015; Rapid and efficient direct conversion of human adult somatic cells into neural stem cells by HMGA2/let-7b. Cell Rep. 10:441–452. DOI: 10.1016/j.celrep.2014.12.038. PMID: 25600877.
Article22. Wang L, Wang L, Huang W, Su H, Xue Y, Su Z, Liao B, Wang H, Bao X, Qin D, He J, Wu W, So KF, Pan G, Pei D. 2013; Generation of integration-free neural progenitor cells from cells in human urine. Nat Methods. 10:84–89. DOI: 10.1038/nmeth.2283. PMID: 23223155.
Article23. Kim SM, Lim KT, Kwak TH, Lee SC, Im JH, Hali S, In Hwang S, Kim D, Hwang J, Kim KP, Chung HJ, Kim JB, Ko K, Chung HM, Lee HT, Schöler HR, Han DW. 2016; Induced neural stem cells from distinct genetic backgrounds exhibit different reprogramming status. Stem Cell Res. 16:460–468. DOI: 10.1016/j.scr.2016.02.025. PMID: 26930613.
Article24. Yamashita T, Liu W, Matsumura Y, Miyagi R, Zhai Y, Kusaki M, Hishikawa N, Ohta Y, Kim SM, Kwak TH, Han DW, Abe K. 2017; Novel therapeutic transplantation of induced neural stem cells for stroke. Cell Transplant. 26:461–467. DOI: 10.3727/096368916X692988. PMID: 27653466. PMCID: PMC5657696.
Article25. Velychko S, Kang K, Kim SM, Kwak TH, Kim KP, Park C, Hong K, Chung C, Hyun JK, MacCarthy CM, Wu G, Schöler HR, Han DW. 2019; Fusion of reprogramming factors alters the trajectory of somatic lineage conversion. Cell Rep. 27:30–39.e4. DOI: 10.1016/j.celrep.2019.03.023. PMID: 30943410.
Article26. Petersen GF, Strappe PM. 2016; Generation of diverse neural cell types through direct conversion. World J Stem Cells. 8:32–46. DOI: 10.4252/wjsc.v8.i2.32. PMID: 26981169. PMCID: PMC4766249.
Article27. Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, Kim WR, Lipton SA, Zhang K, Ding S. 2011; Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci U S A. 108:8299–8304. DOI: 10.1073/pnas.1014041108. PMID: 21525408. PMCID: PMC3100988.
Article28. Xu Y, Wei X, Wang M, Zhang R, Fu Y, Xing M, Hua Q, Xie X. 2013; Proliferation rate of somatic cells affects reprogramming efficiency. J Biol Chem. 288:9767–9778. DOI: 10.1074/jbc.M112.403881. PMID: 23439651. PMCID: PMC3617278.
Article29. Ruiz S, Panopoulos AD, Herrerías A, Bissig KD, Lutz M, Berggren WT, Verma IM, Izpisua Belmonte JC. 2011; A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Curr Biol. 21:45–52. DOI: 10.1016/j.cub.2010.11.049. PMID: 21167714. PMCID: PMC3034649.
Article30. Mirakhori F, Zeynali B, Rassouli H, Shahbazi E, Hashemizadeh S, Kiani S, Salekdeh GH, Baharvand H. 2015; Induction of neural progenitor-like cells from human fibroblasts via a genetic material-free approach. PLoS One. 10:e0135479. DOI: 10.1371/journal.pone.0135479. PMID: 26266943. PMCID: PMC4534403.
Article31. Zheng J, Choi KA, Kang PJ, Hyeon S, Kwon S, Moon JH, Hwang I, Kim YI, Kim YS, Yoon BS, Park G, Lee J, Hong S, You S. 2017; Corrigendum to "A combination of small molecules directly reprograms mouse fibroblasts into neural stem cells" [Biochem. Biophys. Res. Commun. 476 (2016) 42-48]. Biochem Biophys Res Commun. 485:705. DOI: 10.1016/j.bbrc.2017.02.054. PMID: 28237361.
Article32. Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. 2008; Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 451:141–146. DOI: 10.1038/nature06534. PMID: 18157115.
Article33. Sgodda M, Mobus S, Hoepfner J, Sharma AD, Schambach A, Greber B, Ott M, Cantz T. 2013; Improved hepatic differentiation strategies for human induced pluripotent stem cells. Curr Mol Med. 13:842–855. DOI: 10.2174/1566524011313050015. PMID: 23642065.
Article34. Hoch RV, Lindtner S, Price JD, Rubenstein JL. 2015; OTX2 transcription factor controls regional patterning within the medial ganglionic eminence and regional identity of the septum. Cell Rep. 12:482–494. DOI: 10.1016/j.celrep.2015.06.043. PMID: 26166575. PMCID: PMC4512845.
Article35. Gaber ZB, Butler SJ, Novitch BG. 2013; PLZF regulates fibroblast growth factor responsiveness and maintenance of neural progenitors. PLoS Biol. 11:e1001676. DOI: 10.1371/journal.pbio.1001676. PMID: 24115909. PMCID: PMC3792860.
Article36. Mitchell RR, Szabo E, Benoit YD, Case DT, Mechael R, Alamilla J, Lee JH, Fiebig-Comyn A, Gillespie DC, Bhatia M. 2014; Activation of neural cell fate programs toward direct conversion of adult human fibroblasts into tri-potent neural progenitors using OCT-4. Stem Cells Dev. 23:1937–1946. DOI: 10.1089/scd.2014.0023. PMID: 24694094. PMCID: PMC4120813.
Article37. Shimazaki T, Arsenijevic Y, Ryan AK, Rosenfeld MG, Weiss S. 1999; A role for the POU-III transcription factor Brn-4 in the regulation of striatal neuron precursor differentiation. EMBO J. 18:444–456. DOI: 10.1093/emboj/18.2.444. PMID: 9889200. PMCID: PMC1171138.
Article38. Francesconi M, Di Stefano B, Berenguer C, de Andrés-Aguayo L, Plana-Carmona M, Mendez-Lago M, Guillaumet-Adkins A, Rodriguez-Esteban G, Gut M, Gut IG, Heyn H, Lehner B, Graf T. 2019; Single cell RNA-seq identifies the origins of heterogeneity in efficient cell transdifferentiation and reprogramming. Elife. 8:e41627. DOI: 10.7554/eLife.41627. PMID: 30860479. PMCID: PMC6435319.
Article39. Kang X, Yu Q, Huang Y, Song B, Chen Y, Gao X, He W, Sun X, Fan Y. 2015; Effects of integrating and non-integrating reprogramming methods on copy number variation and genomic stability of human induced pluripotent stem cells. PLoS One. 10:e0131128. DOI: 10.1371/journal.pone.0131128. PMID: 26131765. PMCID: PMC4488894.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Concepts of Stem Cell Therapy
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- Induced Pluripotent Stem Cells: Next Generation Stem Cells to Clinical Applications
- Disease-specific pluripotent stem cells
- Modeling of Human Genetic Diseases Via Cellular Reprogramming