Cancer Res Treat.
2020 Apr;52(2):563-572. 10.4143/crt.2019.249.
Association of Body Composition with Long-Term Survival inNon-metastatic Rectal Cancer Patients
- Affiliations
-
- 1Department of Colon and Rectal Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2500341
- DOI: http://doi.org/10.4143/crt.2019.249
Abstract
- Purpose
We evaluated the association of body composition with long-term oncologic outcomes in non-metastatic rectal cancer patients.
Materials and Methods
We included 1,384 patients with stage(y)0-III rectal cancer treated at Asan Medical Center between January 2005 and December 2012. Body composition at diagnosis was measured using abdomino-pelvic computed tomography (CT). Sarcopenia, visceral obesity (VO), and sarcopenic obesity (SO) were defined using CT measured parameters such as skeletal muscle index (total abdominal muscle area, TAMA), visceral fat area (VFA), and VFA/TAMA. Inflammatory status was defined as a neutrophil-lymphocyte ratio of ! 3. Obesity was categorized by body mass index (! 25 kg/m2).
Results
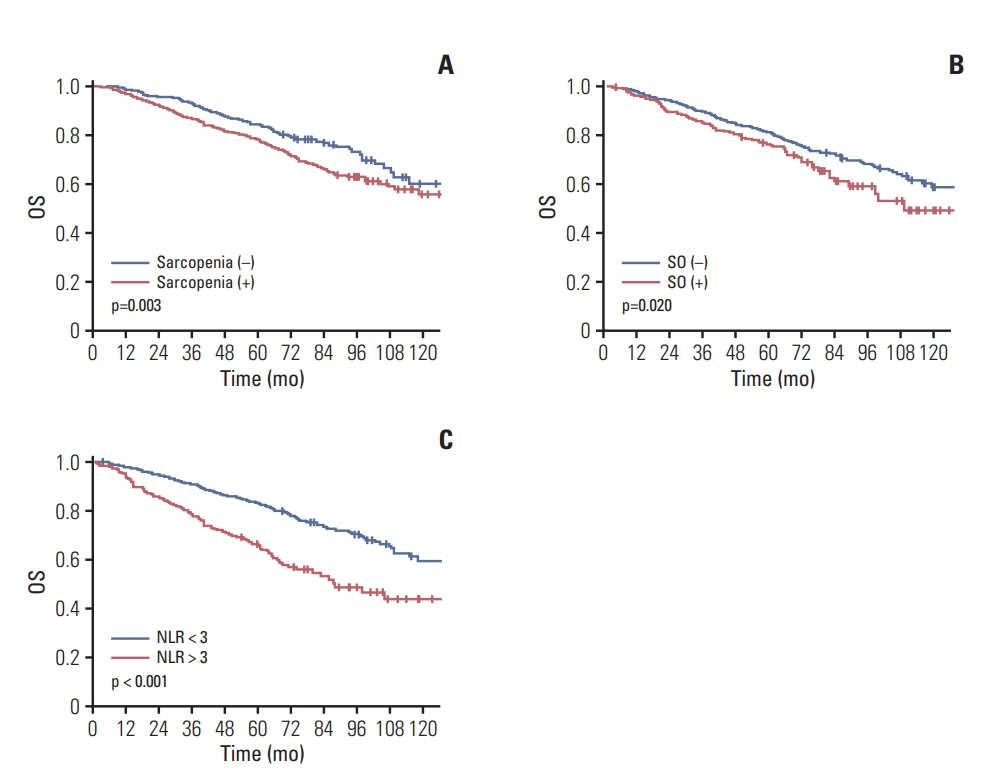
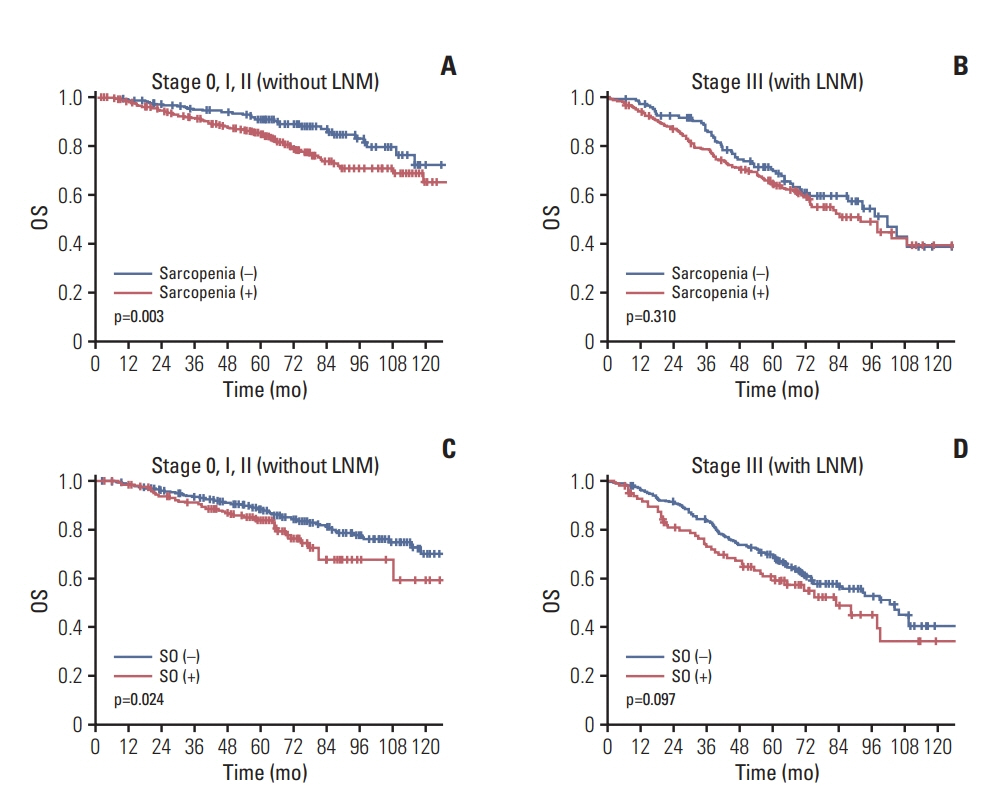
Among the 1,384 patients, 944 (68.2%) had sarcopenia and 307 (22.2%) had SO. The 5-year overall survival (OS) rate was significantly lower in sarcopenic patients (no sarcopenia vs. sarcopenia; 84% vs. 78%, p=0.003) but the 5-year recurrence-free survival (RFS) rate was not different (77.3% vs. 77.9% p=0.957). Patients with SO showed lower 5-year OS (79.1% vs. 75.5% p=0.02) but no difference in 5-year RFS (p=0.957). Sarcopenia, SO, VO, and obesity were not associated with RFS. However, obesity, SO, age, sex, inflammatory status, and tumor stage were confirmed as independent factors associated with OS on multivariate analysis. In subgroup analysis, association of SO with OS was more prominent in patients with (y)p stage 0-2 and no inflammatory status.
Conclusion
The presence of SO and a low body mass index at diagnosis are negatively associated with OS in non-metastatic rectal cancer patients.
Figure
Reference
-
References
1. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015; 22:2663–8.
Article2. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a metaanalysis and systematic review. Eur J Cancer. 2016; 57:58–67.
Article3. Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017; 115:470–9.
Article4. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008; 9:629–35.
Article5. Malietzis G, Johns N, Al-Hassi HO, Knight SC, Kennedy RH, Fearon KC, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016; 263:320–5.
Article6. Black D, Mackay C, Ramsay G, Hamoodi Z, Nanthakumaran S, Park KG, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. 2017; 24:2241–51.
Article7. Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The impact of muscle and adipose tissue on long-term survival in patients with stage I to III colorectal cancer. Dis Colon Rectum. 2019; 62:549–60.
Article8. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012; 107:931–6.
Article9. Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015; 17:O256–64.
Article10. Feliciano EM, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017; 3:e172319.11. Paik KY, Lee IK, Lee YS, Sung NY, Kwon TS. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat. 2014; 46:65–73.
Article12. Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg. 2016; 263:326–36.13. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017; 96:10–5.
Article14. Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013; 100:1523–30.
Article15. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle. 2018; 9:53–9.
Article16. Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018; 29:ii1–9.
Article17. Carneiro IP, Mazurak VC, Prado CM. Clinical implications of sarcopenic obesity in cancer. Curr Oncol Rep. 2016; 18:62.
Article18. Gruber ES, Jomrich G, Tamandl D, Gnant M, Schindl M, Sahora K. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. 2019; 14:e0215915.
Article19. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–7.
Article20. Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011; 104:1288–95.
Article21. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006; 6:772–83.
Article22. Xing SQ, Zhang CG, Yuan JF, Yang HM, Zhao SD, Zhang H. Adiponectin induces apoptosis in hepatocellular carcinoma through differential modulation of thioredoxin proteins. Biochem Pharmacol. 2015; 93:221–31.
Article23. Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle: adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem. 2011; 117:47–56.24. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012; 8:457–65.
Article25. Leitch EF, Chakrabarti M, Crozier JE, McKee RF, Anderson JH, Horgan PG, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007; 97:1266–70.
Article26. Liu Y, He X, Pan J, Chen S, Wang L. Prognostic role of Glasgow prognostic score in patients with colorectal cancer: evidence from population studies. Sci Rep. 2017; 7:6144.
Article27. Broughman JR, Williams GR, Deal AM, Yu H, Nyrop KA, Alston SM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. J Geriatr Oncol. 2015; 6:442–5.
Article28. Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewe KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015; 261:345–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of p53 Overexpression in the Metastasis of Colorectal Cancer
- Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer
- Comparison of long-term oncologic outcomes of stage III colorectal cancer following laparoscopic versus open surgery
- The Effect of Regular Exercise Program on Body Composition and Body Image in Adults Using One Fitness Center
- Factors Affecting Long Term Survival for Metastatic Gastric Cancer Treated with Chemotherapy