J Clin Neurol.
2020 Jan;16(1):9-18. 10.3988/jcn.2020.16.1.9.
Effect of Repetitive Transcranial Magnetic Stimulation on Seizure Frequency and Epileptiform Discharges in Drug-Resistant Epilepsy: A Meta-Analysis
- Affiliations
-
- 1Department of Pharmacology and Psychiatry, All India Institute of Medical Sciences (AIIMS), Bhubaneswar, India. pharm_rituparna@aiimsbhubaneswar.edu.in
- KMID: 2467779
- DOI: http://doi.org/10.3988/jcn.2020.16.1.9
Abstract
- BACKGROUND AND PURPOSE
The role of low-frequency repetitive transcranial stimulation (rTMS) in drug-resistant epilepsy (DRE) has been conflicting and inconclusive in previous clinical trials. This meta-analysis evaluated the efficacy of rTMS on seizure frequency and epileptiform discharges in DRE.
METHODS
A standard meta-analysis protocol was registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO: CRD42018088544). After performing a comprehensive literature search using specific keywords in MEDLINE, the Cochrane database, and the International Clinical Trial Registry Platform (ICTRP), reviewers assessed the eligibility and extracted data from seven relevant clinical trials. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed in the selection, analysis, and reporting of findings. A random-effects model was used to estimate the effect size as the mean difference in seizure frequency and interictal epileptiform discharges between the groups. Quality assessment was performed using a risk-of-bias assessment tool, and a meta-regression was used to identify the variables that probably influenced the effect size.
RESULTS
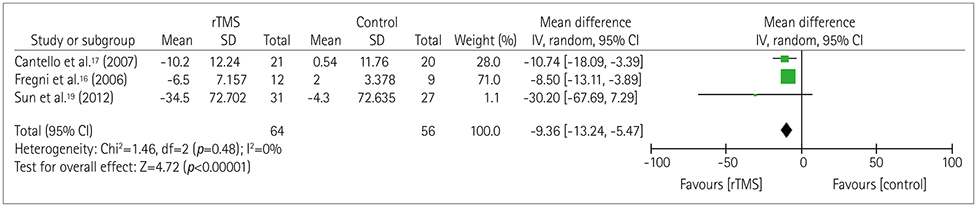
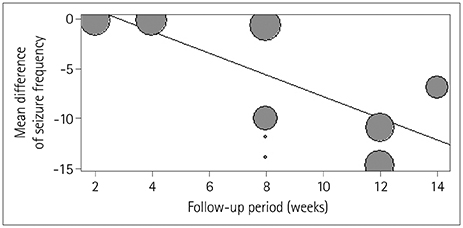
The random-effects model analysis revealed a pooled effect size of −5.96 (95% CI= −8.98 to −2.94), significantly favoring rTMS stimulation (p=0.0001) over the control group with regard to seizure frequency. The overall effect size for interictal epileptiform discharges also significantly favored rTMS stimulation (p < 0.0001), with an overall effect size of −9.36 (95% CI=−13.24 to −5.47). In the meta-regression, the seizure frequency worsened by 2.00±0.98 (mean±SD, p=0.042) for each week-long lengthening of the posttreatment follow-up period, suggesting that rTMS exerts only a short-term effect.
CONCLUSIONS
This meta-analysis shows that rTMS exerts a significant beneficial effect on DRE by reducing both the seizure frequency and interictal epileptiform discharges. However, the meta-regression revealed only an ephemeral effect of rTMS.
MeSH Terms
Figure
Reference
-
1. Lopes da, Blanes W, Kalitzin SN, Parra J, Suffczynski P, Velis DN. Epilepsies as dynamical diseases of brain systems: basic models of the transition between normal and epileptic activity. Epilepsia. 2003; 44 Suppl 12:72–83.
Article2. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010; 51:1069–1077.
Article3. Malhi GS, Loo C, Cahill CM, Lagopoulos J, Mitchell P, Sachdev P. “Getting physical”: the management of neuropsychiatric disorders using novel physical treatments. Neuropsychiatr Dis Treat. 2006; 2:165–179.
Article4. Klooster DC, De Louw AJ, Aldenkamp AP, Besseling RM, Mestrom RM, Carrette S, et al. Technical aspects of neurostimulation: focus on equipment, electric field modeling, and stimulation protocols. Neurosci Biobehav Rev. 2016; 65:113–141.
Article5. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003; 2:145–156.
Article6. Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013; 6:1–13.
Article7. Cincotta M, Borgheresi A, Gambetti C, Balestrieri F, Rossi L, Zaccara G, et al. Suprathreshold 0.3 Hz repetitive TMS prolongs the cortical silent period: potential implications for therapeutic trials in epilepsy. Clin Neurophysiol. 2003; 114:1827–1833.
Article8. Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci. 2015; 9:303.
Article9. Fregni F, Thome-Souza S, Bermpohl F, Marcolin MA, Herzog A, Pascual-Leone A, et al. Antiepileptic effects of repetitive transcranial magnetic stimulation in patients with cortical malformations: an EEG and clinical study. Stereotact Funct Neurosurg. 2005; 83:57–62.
Article10. Daniele O, Brighina F, Piazza A, Giglia G, Scalia S, Fierro B. Low-frequency transcranial magnetic stimulation in patients with cortical dysplasia - a preliminary study. J Neurol. 2003; 250:761–762.
Article11. Joo EY, Han SJ, Chung SH, Cho JW, Seo DW, Hong SB. Antiepileptic effects of low-frequency repetitive transcranial magnetic stimulation by different stimulation durations and locations. Clin Neurophysiol. 2007; 118:702–708.
Article12. Kinoshita M, Ikeda A, Begum T, Yamamoto J, Hitomi T, Shibasaki H. Low-frequency repetitive transcranial magnetic stimulation for seizure suppression in patients with extratemporal lobe epilepsy-a pilot study. Seizure. 2005; 14:387–392.
Article13. Tergau F, Naumann U, Paulus W, Steinhoff BJ. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. 1999; 353:2209.
Article14. Tergau F, Neumann D, Rosenow F, Nitsche MA, Paulus W, Steinhoff B. Can epilepsies be improved by repetitive transcranial magnetic stimulation?--interim analysis of a controlled study. Suppl Clin Neurophysiol. 2003; 56:400–405.15. Theodore WH, Hunter K, Chen R, Vega-Bermudez F, Boroojerdi B, Reeves-Tyer P, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology. 2002; 59:560–562.
Article16. Fregni F, Otachi PT, Do Valle A, Boggio PS, Thut G, Rigonatti SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006; 60:447–455.
Article17. Cantello R, Rossi S, Varrasi C, Ulivelli M, Civardi C, Bartalini S, et al. Slow repetitive TMS for drug-resistant epilepsy: clinical and EEG findings of a placebo-controlled trial. Epilepsia. 2007; 48:366–374.
Article18. Wang X, Yang D, Wang S, Zhao X, Zhang L, Chen Z, et al. Effects of low-frequency repetitive transcranial magnetic stimulation on electroencephalogram and seizure frequency in 15 patients with temporal lobe epilepsy following dipole source localization. Neural Regen Res. 2008; 3:1257–1260.19. Sun W, Mao W, Meng X, Wang D, Qiao L, Tao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia. 2012; 53:1782–1789.
Article20. Seynaeve L, Devroye A, Dupont P, Van Paesschen W. Randomized crossover sham-controlled clinical trial of targeted low-frequency transcranial magnetic stimulation comparing a figure-8 and a round coil to treat refractory neocortical epilepsy. Epilepsia. 2016; 57:141–150.
Article21. Hsu WY, Cheng CH, Lin MW, Shih YH, Liao KK, Lin YY. Antiepileptic effects of low frequency repetitive transcranial magnetic stimulation: a meta-analysis. Epilepsy Res. 2011; 96:231–240.
Article22. Chen R, Spencer DC, Weston J, Nolan SJ. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database Syst Rev. 2016; 8:CD011025.
Article23. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1.
Article24. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
Article25. Cochrane Community. RevMan 5. Cochrane. cited 2018 Apr 21. Available from: https://community.cochrane.org/help/tools-andsoftware/revman-5/revman-5-download.26. Schwarzer G. Meta: an R package for meta-analysis. R News. 2007; 7:40–45.27. Kimiskidis VK, Valentin A, Kälviäinen R. Transcranial magnetic stimulation for the diagnosis and treatment of epilepsy. Curr Opin Neurol. 2014; 27:236–241.
Article28. Bae EH, Theodore WH, Fregni F, Cantello R, Pascual-Leone A, Rotenberg A. An estimate of placebo effect of repetitive transcranial magnetic stimulation in epilepsy. Epilepsy Behav. 2011; 20:355–359.
Article29. Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998; 250:141–144.
Article30. Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010; 588:2291–2304.
Article31. Pereira LS, Müller VT, Da Mota Gomes M, Rotenberg A, Fregni F. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: a systematic review. Epilepsy Behav. 2016; 57:167–176.
Article32. Brasil-Neto JP, De Araújo DP, Teixeira WA, Araújo VP, Boechat-Barros R. Experimental therapy of epilepsy with transcranial magnetic stimulation: lack of additional benefit with prolonged treatment. Arq Neuropsiquiatr. 2004; 62:21–25.
Article33. Sun W, Fu W, Mao W, Wang D, Wang Y. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy. Clin EEG Neurosci. 2011; 42:40–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment-Resistant Depression Entering Remission Following a Seizure during the Course of Repetitive Transcranial Magnetic Stimulation
- Effects of 1 Hz Repetitive Transcranial Magnetic Stimulation on Cortical Excitability and Seizure Reduction in Intractable Neocortical Epilepsy
- Early Augmentation Response with Low-frequency Repetitive Transcranial Magnetic Stimulation in Treatment Resistant Depression
- Analysis of Interictal Epileptiform Discharges in the Benign Childhood Epilepsy with Centrotemporal Spikes: Prediction of Seizure Outcome
- Clinical Efficacy of Repetitive Transcranial Magnetic Stimulation for Treatment of Depression and Latest Trends in TMS Techniques