Acute Crit Care.
2019 Nov;34(4):269-275. 10.4266/acc.2019.00591.
Risk factor, monitoring, and treatment for snakebite induced coagulopathy: a multicenter retrospective study
- Affiliations
-
- 1Department of Surgery, Chinjujeil Hospital, Jinju, Korea. yjjeongs16@naver.com
- 2Department of Surgery, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea.
- KMID: 2467306
- DOI: http://doi.org/10.4266/acc.2019.00591
Abstract
- BACKGROUND
Snakebite can cause various complications, including coagulopathy. The clinical features of snakebite-associated coagulopathy differ from those of disseminated intravascular coagulation (DIC) caused by other diseases and its treatment is controversial.
METHODS
We retrospectively reviewed the medical records of patients hospitalized for snakebite between January 2006 and September 2018.
RESULTS
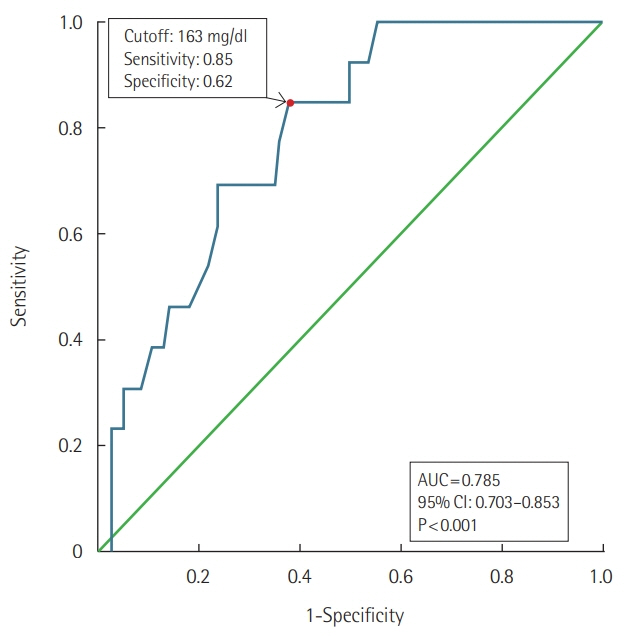
A total of 226 patients were hospitalized due to snakebite. Their median hospital stay was 4.0 days (interquartile range, 2.0 to 7.0 days). Five patients arrived at hospital with shock and one patient died. Twenty-one patients had overt DIC according to the International Society of Thrombosis and Hemostasis scoring system. Two patients developed major bleeding complications. Initial lower cholesterol level at presentation was associated with the development of overt DIC. International normalization ratio (INR) exceeding the laboratory's measurement limit was recorded as late as 4 to 5 days after the bite. Higher antivenom doses (≥18,000 units) and transfusion of fresh frozen plasma (FFP) or cryoprecipitate did not affect prolonged INR duration or hospital stay in the overt DIC patients without bleeding.
CONCLUSIONS
Initial lower cholesterol level may be a risk factor for overt DIC following snakebite. Although patients lack apparent symptoms, the risk of coagulopathy should be assessed for at least 4 to 5 days following snakebite. Higher antivenom doses and transfusion of FFP or cryoprecipitate may be unbeneficial for coagulopathic patients without bleeding.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. Venomous snakes distribution and species risk categories [Internet]. Geneva: World Health Organization;2010. [cited 2019 Aug 28]. Available from: http://apps.who.int/bloodproducts/snakeantivenoms/database/.2. Berling I, Isbister GK. Hematologic effects and complications of snake envenoming. Transfus Med Rev. 2015; 29:82–9.
Article3. Lim H, Kang HG, Kim KH. Antivenom for snake bite in Korea. J Korean Med Assoc. 2013; 56:1091–103.
Article4. Ramos OH, Selistre-de-Araujo HS. Snake venom metalloproteases: structure and function of catalytic and disintegrin domains. Comp Biochem Physiol C Toxicol Pharmacol. 2006; 142:328–46.5. Isbister GK. Procoagulant snake toxins: laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin Thromb Hemost. 2009; 35:93–103.
Article6. Slagboom J, Kool J, Harrison RA, Casewell NR. Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br J Haematol. 2017; 177:947–59.
Article7. Lee BJ, Hong SI, Kim HS, Kim TH, Lee JH, Ryu BY, et al. Hematological features of coagulopathy and the efficacy of antivenin therapy for a Korean snakebite. J Korean Surg Soc. 2007; 72:18–26.8. Isbister GK, Jayamanne S, Mohamed F, Dawson AH, Maduwage K, Gawarammana I, et al. A randomized controlled trial of fresh frozen plasma for coagulopathy in Russell’s viper (Daboia russelii) envenoming. J Thromb Haemost. 2017; 15:645–54.
Article9. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation: British Committee for Standards in Haematology. Br J Haematol. 2009; 145:24–33.10. Winkler E, Chovers M, Almog S, Pri-Chen S, Rotenberg M, Tirosh M, et al. Decreased serum cholesterol level after snake bite (Vipera palaestinae) as a marker of severity of envenomation. J Lab Clin Med. 1993; 121:774–8.11. Kim JS, Yang JW, Kim MS, Han ST, Kim BR, Shin MS, et al. Coagulopathy in patients who experience snakebite. Korean J Intern Med. 2008; 23:94–9.
Article12. Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008; 5:e218.
Article13. Shim JH, Son YJ, Lee SS, Park KS, Oh HB, Park YD. Ecological study on poisonous snake and investigation of the venom characteristics, snakebiting frequency in Korea. Korean J Environ Ecol. 1998; 12:58–77.14. Lu Q, Clemetson JM, Clemetson KJ. Snake venoms and hemostasis. J Thromb Haemost. 2005; 3:1791–9.
Article15. McCleary RJ, Kini RM. Snake bites and hemostasis/thrombosis. Thromb Res. 2013; 132:642–6.
Article16. White J. Snake venoms and coagulopathy. Toxicon. 2005; 45:951–67.
Article17. Warrell DA. Snake bite. Lancet. 2010; 375:77–88.
Article18. Churchman A, O’Leary MA, Buckley NA, Page CB, Tankel A, Gavaghan C, et al. Clinical effects of red-bellied black snake (Pseudechis porphyriacus) envenoming and correlation with venom concentrations: Australian Snakebite Project (ASP-11). Med J Aust. 2010; 193:696–700.19. Maduwage K, Isbister GK. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl Trop Dis. 2014; 8:e3220.
Article20. Isbister GK. Antivenom efficacy or effectiveness: the Australian experience. Toxicology. 2010; 268:148–54.
Article21. Isbister GK, Scorgie FE, O’Leary MA, Seldon M, Brown SG, Lincz LF, et al. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J Thromb Haemost. 2010; 8:2504–13.
Article22. Isbister GK, Duffull SB, Brown SG, ASP Investigators. Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM. 2009; 102:563–8.
Article23. Mion G, Larréché S, Benois A, Petitjeans F, Puidupin M. Hemostasis dynamics during coagulopathy resulting from Echis envenomation. Toxicon. 2013; 76:103–9.
Article24. Jelinek GA, Smith A, Lynch D, Celenza A, Irving I, Michalopoulos N, et al. The effect of adjunctive fresh frozen plasma administration on coagulation parameters and survival in a canine model of antivenom-treated brown snake envenoming. Anaesth Intensive Care. 2005; 33:36–40.
Article25. Tibballs J. Fresh frozen plasma after brown snake bite: helpful or harmful? Anaesth Intensive Care. 2005; 33:13–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk factor, monitoring, and treatment for snakebite induced coagulopathy: a multicenter retrospective study
- Coagulopathy in patients who experience snakebite
- Availability of erythrocyte sedimentation rate as a predictor of venom induced coagulopathy in patients with snakebite
- Hematological Features of Coagulopathy and the Efficacy of Antivenin Therapy for a Korean Snakebite
- Usefulness of delta neutrophil index for early prediction of overt disseminated intravascular coagulopathy in patients with venomous snakebite