Yonsei Med J.
2016 Jan;57(1):232-237. 10.3349/ymj.2016.57.1.232.
Relationship between Preoperative 18F-Fluorodeoxyglucose Uptake and Epidermal Growth Factor Receptor Status in Primary Colorectal Cancer
- Affiliations
-
- 1Department of Nuclear Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. hshwang@hallym.or.kr
- 2Department of Surgery, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 3Department of Pathology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 4Graduate School of Medicine, Yonsei University, Seoul, Korea.
- KMID: 2466375
- DOI: http://doi.org/10.3349/ymj.2016.57.1.232
Abstract
- PURPOSE
Both 18F-fluorodeoxyglucose (18F-FDG) uptake and epidermal growth factor receptor (EGFR) status are prognostic variables of colorectal cancer (CRC). The aim of this study was to investigate a possible association between 18F-FDG uptake on preoperative positron emission tomography/computed tomography (PET/CT) and EGFR status in primary CRC.
MATERIALS AND METHODS
Records of 132 patients (66 men and 66 women; mean age=67.1+/-11.1 years) who underwent 18F-FDG PET/CT for CRC staging and subsequent bowel resection were reviewed. In primary lesions, 18F-FDG uptake was semiquantitatively evaluated in terms of maximum standardized uptake value (SUVmax), and EGFR status was determined by immunohistochemistry. Associations of clinicopathological parameters and EGFR status were analyzed by Pearson's chi-square test, multiple logistic regression, and receiver operating characteristic curves.
RESULTS
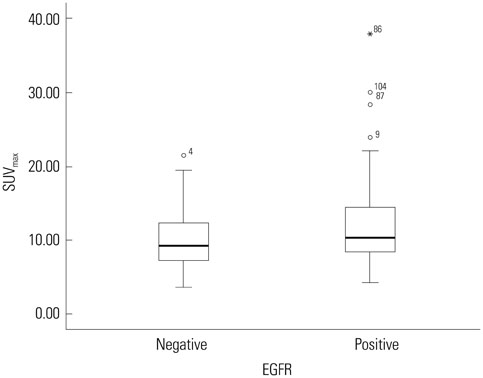
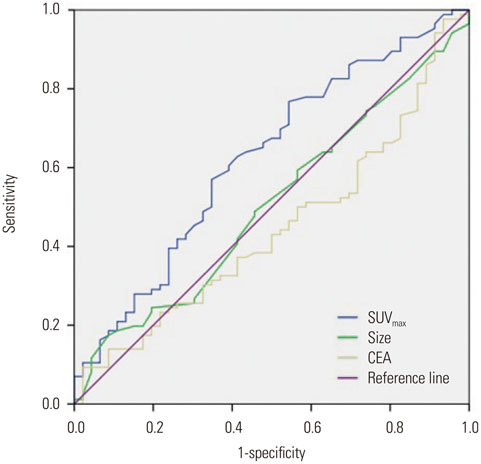
Eighty-six patients (65.2%) showed EGFR expression. SUVmax was significantly lower in EGFR-negative tumors than in EGFR-expressing tumors (10.0+/-4.2 vs. 12.1+/-2.1; p=0.012). It was the only significant parameter correlated with EGFR expression (odds ratio=2.457; relative risk=2.013; p=0.038). At the SUVmax threshold of 7.5, the sensitivity and specificity for predicting EGFR expression were 84.9% and 40.4%, respectively (area under the curve=0.624; p=0.019).
CONCLUSION
Preoperative 18F-FDG uptake is slightly correlated with EGFR status in primary CRC. Preoperative SUVmax of 18F-FDG may have a limited role in predicting EGFR expression in such tumors because of its poor specificity.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Colorectal Neoplasms/metabolism/pathology/*radiography/*radionuclide imaging
Female
Fluorodeoxyglucose F18/*pharmacokinetics
Humans
Immunohistochemistry
Male
Middle Aged
Multimodal Imaging/*methods
Neoplasm Staging
Positron-Emission Tomography/*methods
Predictive Value of Tests
Prognosis
ROC Curve
Radiopharmaceuticals/*pharmacokinetics
Receptor, Epidermal Growth Factor/*metabolism
Sensitivity and Specificity
Fluorodeoxyglucose F18
Receptor, Epidermal Growth Factor
Radiopharmaceuticals
Figure
Reference
-
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
Article2. Iyer RB, Silverman PM, DuBrow RA, Charnsangavej C. Imaging in the diagnosis, staging, and follow-up of colorectal cancer. AJR Am J Roentgenol. 2002; 179:3–13.
Article3. Filippone A, Ambrosini R, Fuschi M, Marinelli T, Genovesi D, Bonomo L. Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography--initial experience. Radiology. 2004; 231:83–90.
Article4. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004; 54:8–29.
Article5. Grizzle WE, Manne U, Jhala NC, Weiss HL. Molecular characterization of colorectal neoplasia in translational research. Arch Pathol Lab Med. 2001; 125:91–98.
Article6. Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011; 6:479–507.
Article7. Yasui W, Sumiyoshi H, Hata J, Kameda T, Ochiai A, Ito H, et al. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 1988; 48:137–141.8. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004; 351:337–345.
Article9. Kopp R, Rothbauer E, Ruge M, Arnholdt H, Spranger J, Muders M, et al. Clinical implications of the EGF receptor/ligand system for tumor progression and survival in gastrointestinal carcinomas: evidence for new therapeutic options. Recent Results Cancer Res. 2003; 162:115–132.
Article10. Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993; 71:2454–2460.
Article11. Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001; 92:1331–1346.
Article12. Park IJ, Kim HC, Yu CS, Ryu MH, Chang HM, Kim JH, et al. Efficacy of PET/CT in the accurate evaluation of primary colorectal carcinoma. Eur J Surg Oncol. 2006; 32:941–947.
Article13. Herbertson RA, Scarsbrook AF, Lee ST, Tebbutt N, Scott AM. Established, emerging and future roles of PET/CT in the management of colorectal cancer. Clin Radiol. 2009; 64:225–237.
Article14. Choi EK, Yoo IeR, Park HL, Choi HS, Han EJ, Kim SH, et al. Value of Surveillance (18)F-FDG PET/CT in Colorectal Cancer: Comparison with Conventional Imaging Studies. Nucl Med Mol Imaging. 2012; 46:189–195.
Article15. Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001; 42:5 Suppl. 1S–93S.16. de Geus-Oei LF, Ruers TJ, Punt CJ, Leer JW, Corstens FH, Oyen WJ. FDG-PET in colorectal cancer. Cancer Imaging. 2006; 6:S71–S81.
Article17. Kwon MJ, Kim DH, Park HR, Shin HS, Kwon JH, Lee DJ, et al. Frequent hepatocyte growth factor overexpression and low frequency of c-Met gene amplification in human papillomavirus-negative tonsillar squamous cell carcinoma and their prognostic significances. Hum Pathol. 2014; 45:1327–1338.
Article18. Delbeke D, Martin WH. PET and PET-CT for evaluation of colorectal carcinoma. Semin Nucl Med. 2004; 34:209–223.
Article19. Figueiras RG, Goh V, Padhani AR, Naveira AB, Caamaño AG, Martin CV. The role of functional imaging in colorectal cancer. AJR Am J Roentgenol. 2010; 195:54–66.
Article20. Kostakoglu L, Agress H Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics. 2003; 23:315–340.
Article21. Tatlidil R, Jadvar H, Bading JR, Conti PS. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology. 2002; 224:783–787.
Article22. Abdel-Nabi H, Doerr RJ, Lamonica DM, Cronin VR, Galantowicz PJ, Carbone GM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998; 206:755–760.
Article23. Mukai M, Sadahiro S, Yasuda S, Ishida H, Tokunaga N, Tajima T, et al. Preoperative evaluation by whole-body 18F-fluorodeoxyglucose positron emission tomography in patients with primary colorectal cancer. Oncol Rep. 2000; 7:85–87.24. Gu J, Yamamoto H, Fukunaga H, Danno K, Takemasa I, Ikeda M, et al. Correlation of GLUT-1 overexpression, tumor size, and depth of invasion with 18F-2-fluoro-2-deoxy-D-glucose uptake by positron emission tomography in colorectal cancer. Dig Dis Sci. 2006; 51:2198–2205.
Article25. Na SJ, Chung YA, Maeng LS, Kim KJ, Sohn KM, Kim SH, et al. Comparison between FDG uptake and pathologic or immunohistochemical parametersin pre-operative PET/CT scan of patient with primary colorectal cancer. Nucl Med Mol Imaging. 2009; 43:557–564.26. Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest New Drugs. 1999; 17:259–269.27. Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995; 95:1897–1905.
Article28. Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008; 13:385–393.
Article29. Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015; 148:88–99.
Article30. Xu Q, Xu AT, Zhu MM, Tong JL, Xu XT, Ran ZH. Predictive and prognostic roles of BRAF mutation in patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a meta-analysis. J Dig Dis. 2013; 14:409–416.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amplification of epidermal growth factor receptor gene in primary cervical cancer
- Amplification of epidermal growth factor receptor gene in primary cervical cancer
- Simultaneous Elevation of Epidermal Growth Factor Receptor and Transforming Growth Factor - alpha in the Serum of Colorectal Cancer Patients
- Prognostic significance of epidermal growth factor receptor expression in human gastric carcinoma
- The Prognostic Value of Epidermal Growth Factor Receptor in Primary Breast Cancer