Korean J Radiol.
2016 Dec;17(6):882-892. 10.3348/kjr.2016.17.6.882.
Transcatheter Arterial Chemoembolization Plus ¹³¹I-Labelled Metuximab versus Transcatheter Arterial Chemoembolization Alone in Intermediate/Advanced Stage Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Liver Surgery, Liver Transplantation Division, West China Hospital, Sichuan University, Chengdu 610041, China. huangjiweimd@hotmail.com
- 2Department of Dermatovenereology, West China Hospital, Sichuan University, Chengdu 610041, China.
- KMID: 2466285
- DOI: http://doi.org/10.3348/kjr.2016.17.6.882
Abstract
OBJECTIVE
The aim of the study was to compare transcatheter arterial chemoembolization (TACE) plus ¹³¹I-labelled metuximab with TACE alone for hepatocellular carcinoma (HCC).
MATERIALS AND METHODS
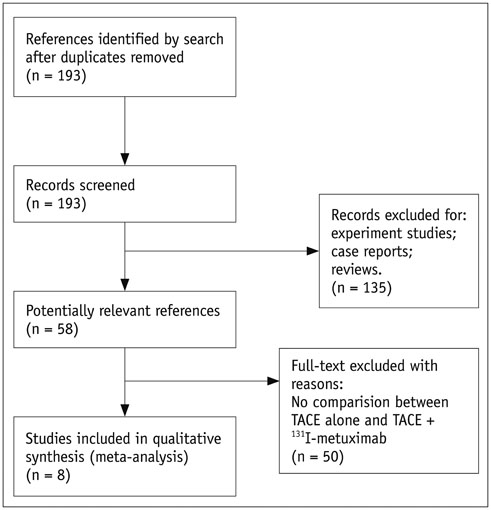
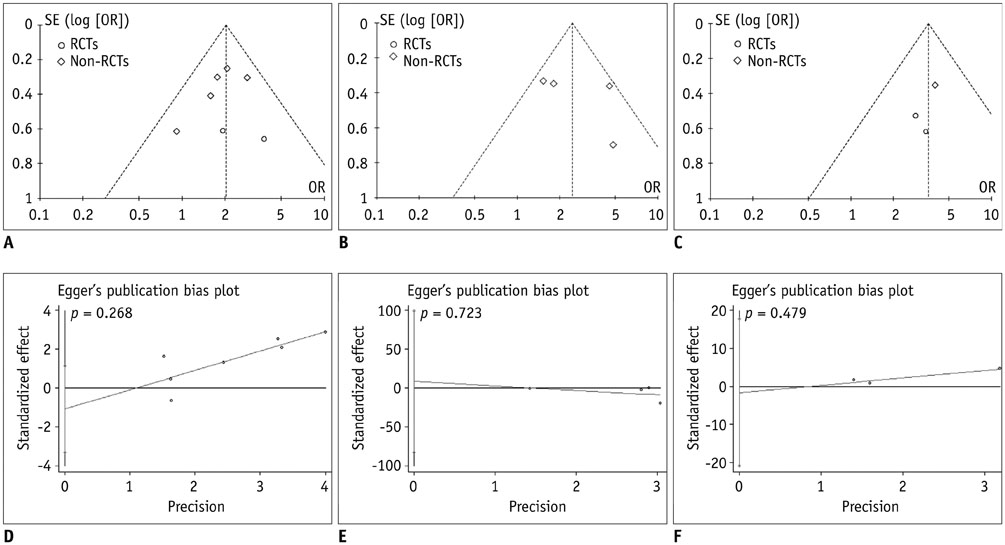
A comprehensive search was conducted in PubMed, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Chinese BioMedical Literature Database with published date from the earliest to February 29th, 2016. No language restrictions were applied, but only prospective randomized controlled trials (RCTs) or non-RCTs were eligible for a full-text review. The primary outcome was the overall survival (OS) and effective rate (the rate of partial atrophy or complete clearance of the tumor lesion). The odds ratios (ORs) were combined using either the fixed-effects model or random-effects model.
RESULTS
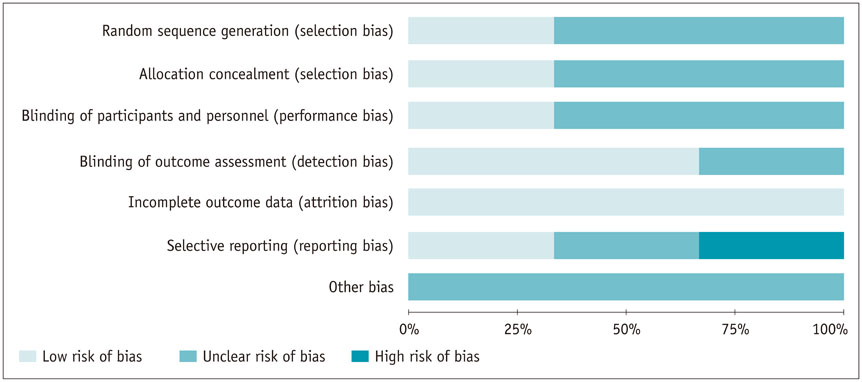
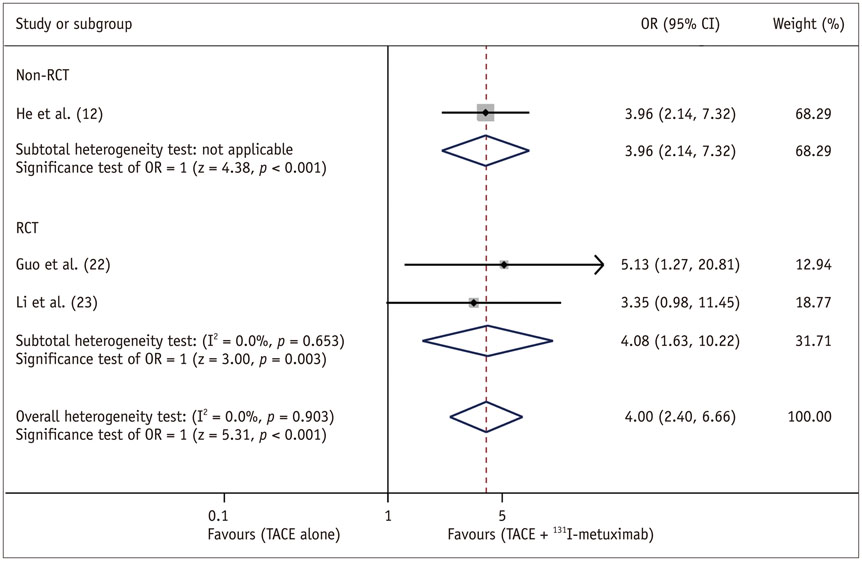
Eight trials (3 RCTs and 5 non-RCTs) were included, involving a total of 1121 patients. Patients receiving combined therapy of TACE plus ¹³¹I-labelled metuximab showed significant improvement in effective rate {OR = 4.00, (95% confidence interval [CI]: 2.40-6.66), p < 0.001}, 1-year OS (OR = 2.03 [95% CI: 1.55-2.67], p < 0.001) and 2-year OS (OR = 2.57 [95% CI: 1.41-4.66], p = 0.002].
CONCLUSION
TACE plus ¹³¹I-labelled metuximab is more beneficial for treating advanced HCCs than TACE alone in terms of tumor response and OS. Large, multi-center, and blinded randomized trials are required to confirm these findings.
Keyword
MeSH Terms
-
Antibodies, Monoclonal/*therapeutic use
Antineoplastic Agents/*therapeutic use
Carcinoma, Hepatocellular/mortality/pathology/*therapy
*Chemoembolization, Therapeutic/adverse effects
Combined Modality Therapy
Databases, Factual
Humans
Liver Neoplasms/mortality/pathology/*therapy
Neoplasm Staging
Radioimmunotherapy
Radiopharmaceuticals/chemistry
Survival Rate
Antibodies, Monoclonal
Antineoplastic Agents
Radiopharmaceuticals
Figure
Reference
-
1. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012; 62:394–399.2. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.3. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003; 37:429–442.4. Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005; 40:225–235.5. Kamran AU, Liu Y, Li FE, Liu S, Wu JL, Zhang YW. Transcatheter Arterial Chemoembolization with gelatin sponge microparticles treated for BCLC stage B hepatocellular carcinoma: a Single Center Retrospective Study. Medicine (Baltimore). 2015; 94:e2154.6. Imai Y, Chikayama T, Nakazawa M, Watanabe K, Ando S, Mizuno Y, et al. Usefulness of miriplatin as an anticancer agent for transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Gastroenterol. 2012; 47:179–186.7. Liu KD, Tang ZY, Bao YM, Lu JZ, Qian F, Yuan AN, et al. Radioimmunotherapy for hepatocellular carcinoma (HCC) using 131I-anti HCC isoferritin IgG: preliminary results of experimental and clinical studies. Int J Radiat Oncol Biol Phys. 1989; 16:319–323.8. Duan CL, Hou GH, Liu YP, Liang T, Song J, Han JK, et al. Tumor vascular homing endgolin-targeted radioimmunotherapy in hepatocellular carcinoma. Tumour Biol. 2014; 35:12205–12215.9. Fujiwara K, Koyama K, Suga K, Ikemura M, Saito Y, Hino A, et al. A (90)Y-labelled anti-ROBO1 monoclonal antibody exhibits antitumour activity against hepatocellular carcinoma xenografts during ROBO1-targeted radioimmunotherapy. EJNMMI Res. 2014; 4:29.10. Chen ZN, Mi L, Xu J, Song F, Zhang Q, Zhang Z, et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: clinical phase I/II trials. Int J Radiat Oncol Biol Phys. 2006; 65:435–444.11. Xu J, Shen ZY, Chen XG, Zhang Q, Bian HJ, Zhu P, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology. 2007; 45:269–276.12. He Q, Lu WS, Liu Y, Guan YS, Kuang AR. 131I-labelled metuximab combined with chemoembolization for unresectable hepatocellular carcinoma. World J Gastroenterol. 2013; 19:9104–9110.13. Wang GP, Feng XS, Shan TY, Sun JT. Clinical study of transcatheter arterial chemoembolization and hepatic perfusion with 131I metuximad injection in treatment of hepatocellular carcinoma [abstract in English]. Chin J Cancer Prev Treat. 2009; 16:1022–1024.14. Higgins JPT, Green S. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. updated March 2011. Accessed January 30, 2016. Available at: http://www.handbook.cochrane.org.15. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010; 8:336–341.16. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.18. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed December 20, 2015. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.19. Wu L, Yang YF, Ge NJ, Shen SQ, Liang J, Wang Y, et al. Hepatic arterial iodine-131-labeled metuximab injection combined with chemoembolization for unresectable hepatocellular carcinoma: interim safety and survival data from 110 patients. Cancer Biother Radiopharm. 2010; 25:657–663.20. Wu L, Yang YF, Ge NJ, Shen SQ, Liang J, Wang Y, et al. Hepatic artery injection of 131I-labelled metuximab combined with chemoembolization for intermediate hepatocellular carcinoma: a prospective nonrandomized study. Eur J Nucl Med Mol Imaging. 2012; 39:1306–1315.21. Ma J, Wang JH. 131I-labelled-metuximab plus transarterial chemoembolization in combination therapy for unresectable hepatocellular carcinoma: results from a multicenter phase IV clinical study. Asian Pac J Cancer Prev. 2015; 16:7441–7447.22. Guo XD, Sun T, Li SS. The effect of iodine[131I] metuximab injection combined with TACE on primary liver cancer [abstract in English]. China J Mod Med. 2011; 21:1206–1208.23. Li Z, Zhou JX, Ren JZ, Zhang WJ, Han XW. [Clinical value of iodine [131I] metuximab infusion combined with TACE for treatment of patients with post-intervention relapse of mid or advanced stage hepatocellular carcinoma]. Zhonghua Gan Zang Bing Za Zhi. 2013; 21:728–733.24. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359:1734–1739.25. Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009; 373:614–616.26. Kuo YC, Chiu YM, Shih WP, Yu HW, Chen CW, Wong PF, et al. Volumetric intensity-modulated Arc (RapidArc) therapy for primary hepatocellular carcinoma: comparison with intensity-modulated radiotherapy and 3-D conformal radiotherapy. Radiat Oncol. 2011; 6:76.27. Zhang Z, Bian H, Feng Q, Mi L, Mo T, Kuang A, et al. Biodistribution and localization of iodine-131-labeled metuximab in patients with hepatocellular carcinoma. Cancer Biol Ther. 2006; 5:318–322.28. Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003; 54:371–389.29. Li J, Huang Q, Long X, Zhang J, Huang X, Aa J, et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol. 2015; 63:1378–1389.30. Dai D, Xu W, Liu J, Zhu L, Zhu X, Ma X. Safety and efficacy of a peripheral intravenous bolus of Licartin for the treatment of advanced hepatocellular carcinoma. Exp Ther Med. 2013; 6:1417–1422.31. Furtado R, Crawford M, Sandroussi C. Systematic review and meta-analysis of adjuvant i(131) lipiodol after excision of hepatocellular carcinoma. Ann Surg Oncol. 2014; 21:2700–2707.32. Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015; 1:756–765.33. Mathurin P, Raynard B, Dharancy S, Kirzin S, Fallik D, Pruvot FR, et al. Meta-analysis: evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinoma. Aliment Pharmacol Ther. 2003; 17:1247–1261.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Fatal Case of Pulmonary Embolism after Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma
- Pirarubicin, UFT, Leucovorin Chemotherapy in Non-embolizable and Transcatheter Arterial Chemoembolization-Failed Hepatocellular Carcinoma Patients; A Phase II Clinical Study
- Rupture of hepatocellular carcinoma after transcatheter arterial chemoembolization: A case report
- Hepatocellular Carcinoma Extending to the Inferior Vena Cava and Right Atrium-A Case Report of 4 Years Survival after Repeated Transcatheter Arterial Chemoembolization Therapy -
- Rupture of Hepatocellular Carcinoma Following Transcatheter Arterial Chemoembolization: A Case Report