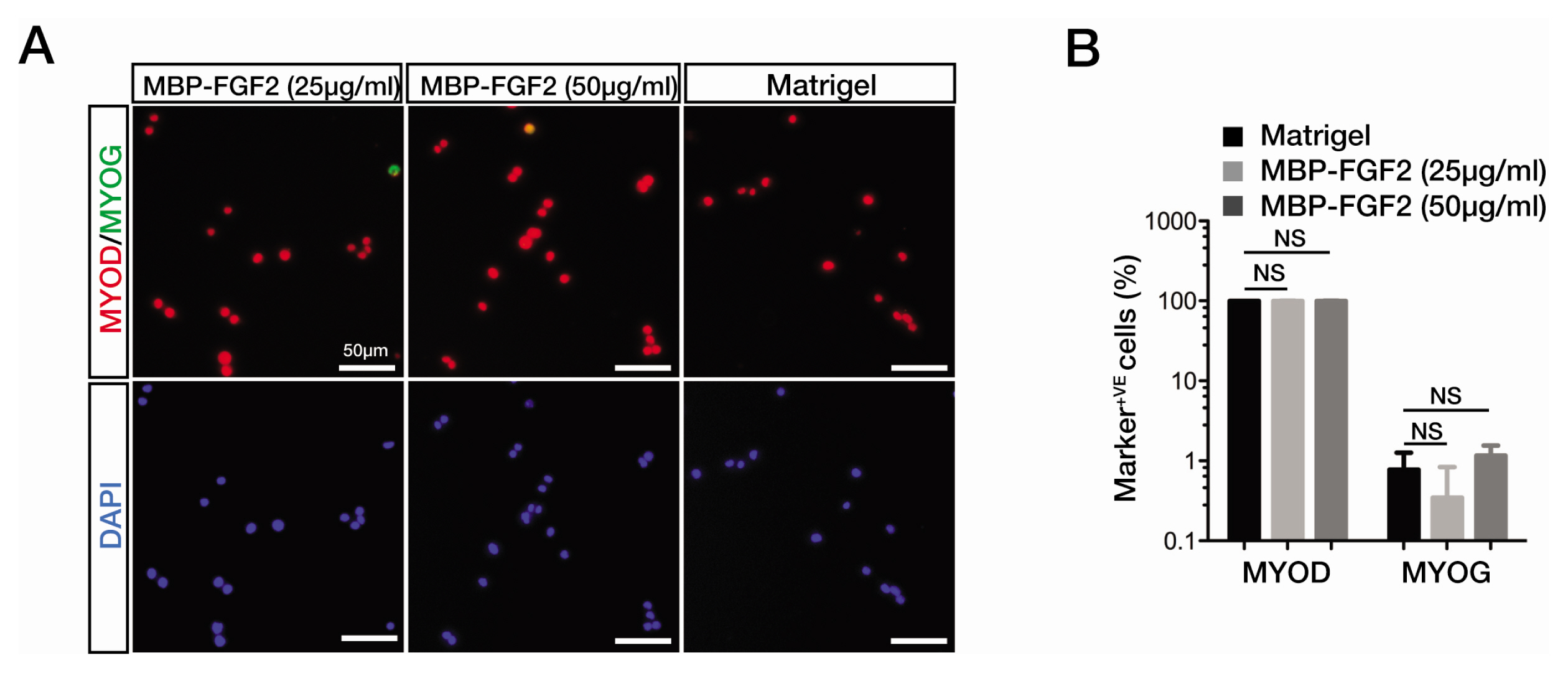
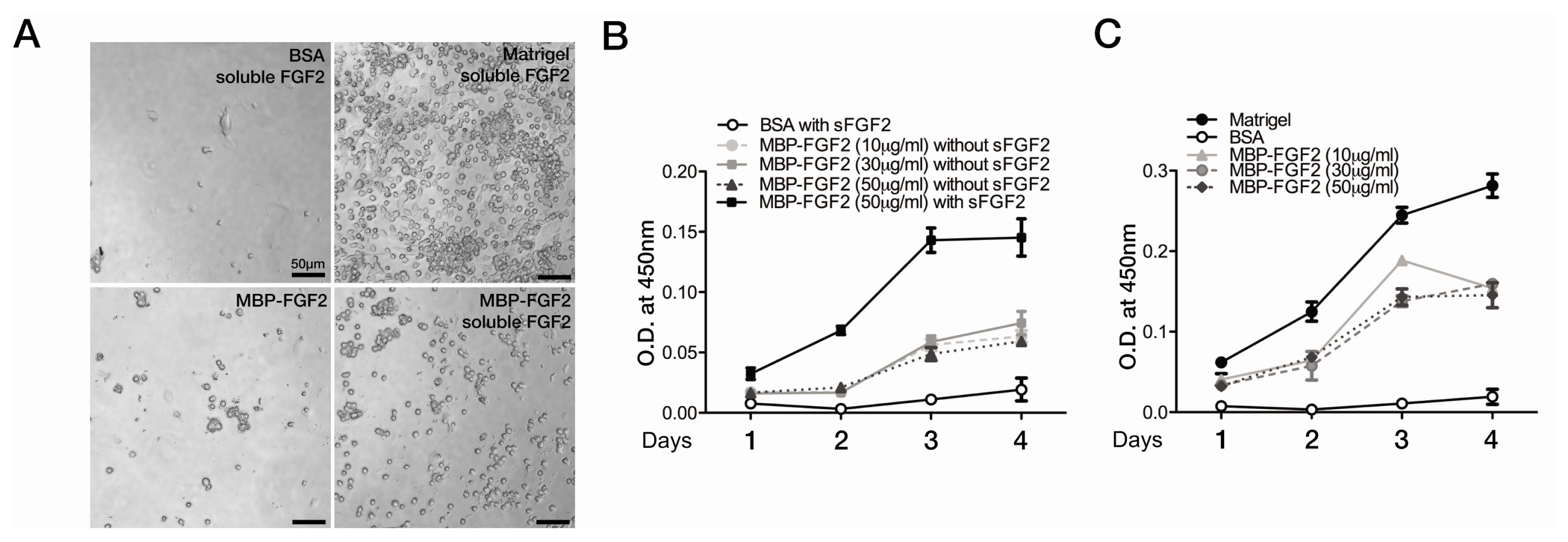
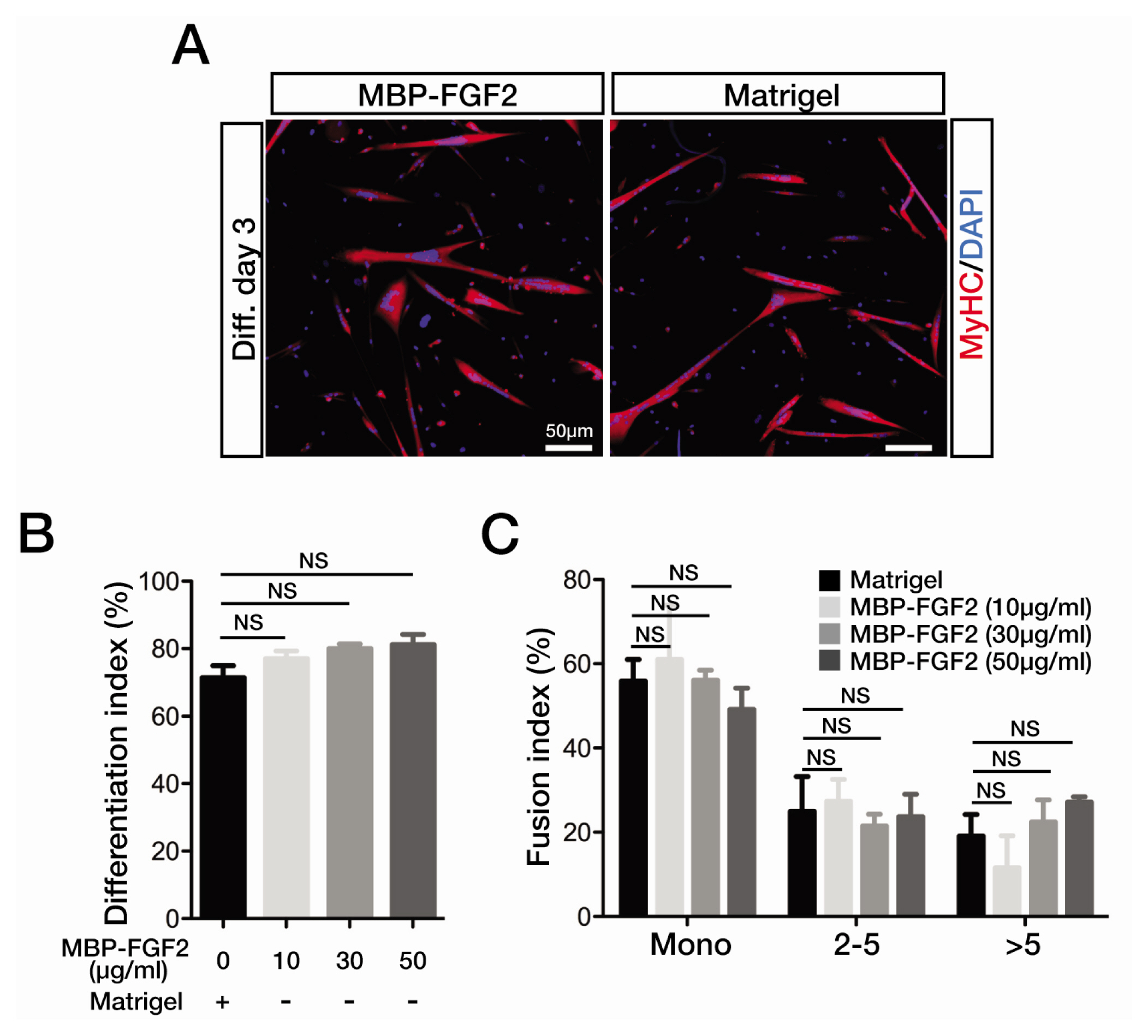
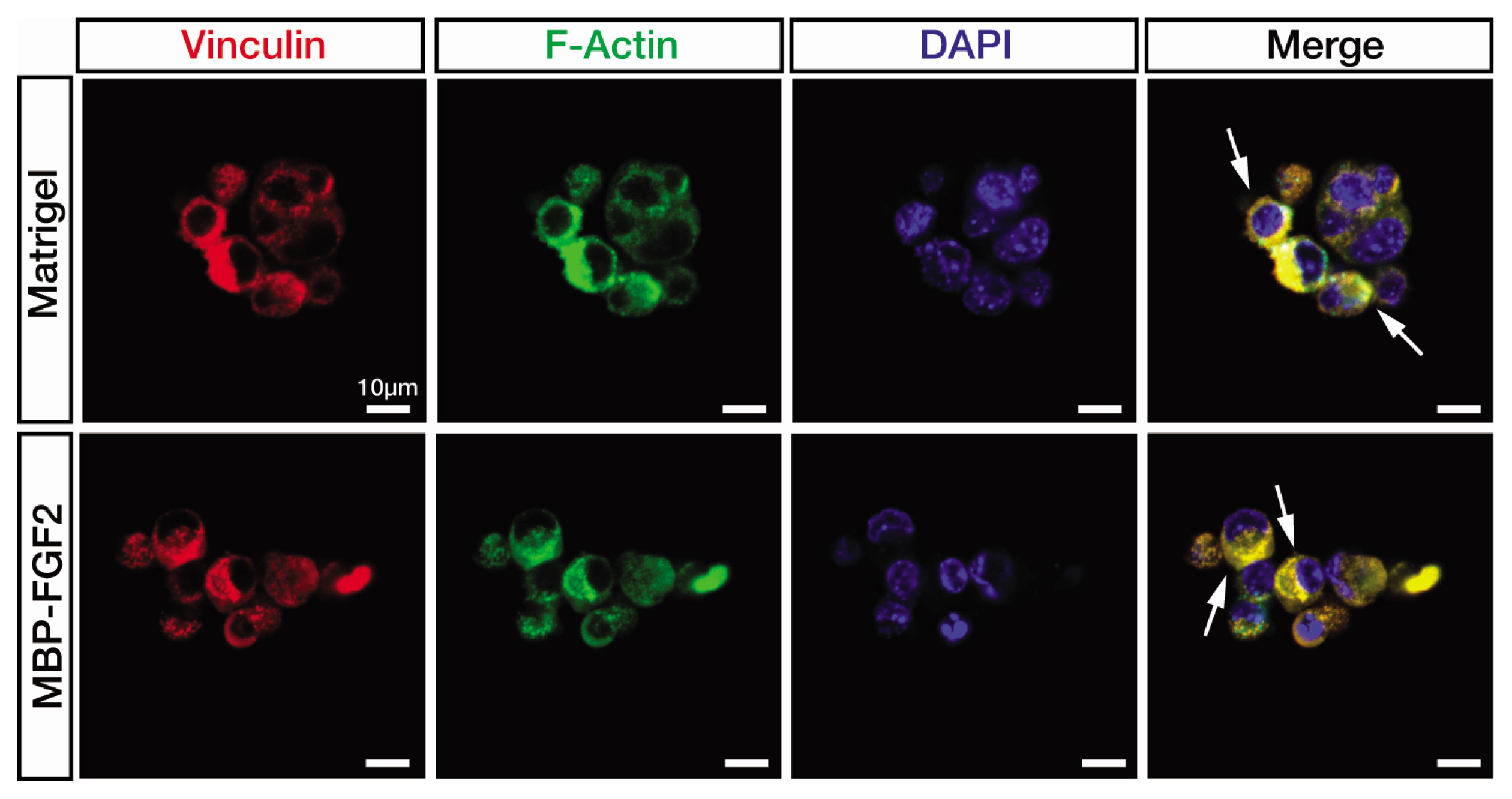
Int J Stem Cells.
2019 Jul;12(2):360-366. 10.15283/ijsc18125.
MBP-FGF2-Immobilized Matrix Maintains Self-Renewal and Myogenic Differentiation Potential of Skeletal Muscle Stem Cells
- Affiliations
-
- 1Soonchunhyang Institute of Medi-bio Science, Soon Chun Hyang University, Cheonan, Korea. jkyoon@sch.ac.kr, yshwang0428@sch.ac.kr
- 2Department of Integrated Biomedical Science, Graduate School, Soon Chun Hyang University, Asan, Korea.
- 3Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel.
- 4Center for Biomaterials, Biomedical Research Institute, Korea Institute of Science and Technology, Seoul, Korea. skimbrc@kist.re.kr
- KMID: 2465905
- DOI: http://doi.org/10.15283/ijsc18125
Abstract
- The robust capacity of skeletal muscle stem cells (SkMSCs, or satellite cells) to regenerate into new muscles in vivo has offered promising therapeutic options for the treatment of degenerative muscle diseases. However, the practical use of SkMSCs to treat muscle diseases is limited, owing to their inability to expand in vitro under defined cultivation conditions without loss of engraftment efficiency. To develop an optimal cultivation condition for SkMSCs, we investigated the behavior of SkMSCs on synthetic maltose-binding protein (MBP)-fibroblast growth factor 2 (FGF2)-immobilized matrix in vitro. We found that the chemically well-defined, xeno-free MBP-FGF2-immobilized matrix effectively supports SkMSC growth without reducing their differentiation potential in vitro. Our data highlights the possible application of the MBP-FGF2 matrix for SkMSC expansion in vitro.
MeSH Terms
Figure
Reference
-
References
1. Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003; 35:1151–1156. DOI: 10.1016/S1357-2725(03)00042-6. PMID: 12757751.
Article2. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013; 93:23–67. DOI: 10.1152/physrev.00043.2011. PMID: 23303905. PMCID: PMC4073943.
Article3. Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat. 2003; 203:89–99. DOI: 10.1046/j.1469-7580.2003.00195.x. PMID: 12892408. PMCID: PMC1571137.
Article4. Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000; 218:115–124. DOI: 10.1006/dbio.1999.9565. PMID: 10656756.
Article5. Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008; 14:82–91. DOI: 10.1016/j.molmed.2007.12.004. PMID: 18218339.
Article6. Charville GW, Cheung TH, Yoo B, Santos PJ, Lee GK, Shrager JB, Rando TA. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Reports. 2015; 5:621–632. DOI: 10.1016/j.stemcr.2015.08.004. PMID: 26344908. PMCID: PMC4624935.
Article7. Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015; 56:1–8. DOI: 10.3109/03008207.2014.947369. PMID: 25047058. PMCID: PMC4464813.
Article8. Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012; 30:232–242. DOI: 10.1002/stem.773. PMID: 22045613. PMCID: PMC3384696.
Article9. Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells. 2012; 30:1971–1984. DOI: 10.1002/stem.1158. PMID: 22730231. PMCID: PMC3465801.
Article10. Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010; 329:1078–1081. DOI: 10.1126/science.1191035. PMID: 20647425. PMCID: PMC2929271.
Article11. Han WM, Anderson SE, Mohiuddin M, Barros D, Nakhai SA, Shin E, Amaral IF, Pêgo AP, García AJ, Jang YC. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci Adv. 2018; 4:eaar4008. DOI: 10.1126/sciadv.aar4008. PMID: 30116776. PMCID: PMC6093653.
Article12. Kang JM, Han M, Park IS, Jung Y, Kim SH, Kim SH. Adhesion and differentiation of adipose-derived stem cells on a substrate with immobilized fibroblast growth factor. Acta Biomater. 2012; 8:1759–1767. DOI: 10.1016/j.actbio.2012.01.005. PMID: 22285427.
Article13. Kang J, Park HM, Kim YW, Kim YH, Varghese S, Seok HK, Kim YG, Kim SH. Control of mesenchymal stem cell phenotype and differentiation depending on cell adhesion mechanism. Eur Cell Mater. 2014; 28:387–403. DOI: 10.22203/eCM.v028a27. PMID: 25422949.
Article14. Kim JH, Park Y, Jung Y, Kim SH, Kim SH. Combinatorial therapy with three-dimensionally cultured adipose-derived stromal cells and self-assembling peptides to enhance angiogenesis and preserve cardiac function in infarcted hearts. J Tissue Eng Regen Med. 2017; 11:2816–2827. DOI: 10.1002/term.2181. PMID: 27256923.
Article15. Motohashi N, Asakura Y, Asakura A. Isolation, culture, and transplantation of muscle satellite cells. J Vis Exp. 2014; Apr. 8. [Epub]. DOI: 10.3791/50846. PMID: 24747722. PMCID: PMC4131689.
Article16. Michailovici I, Harrington HA, Azogui HH, Yahalom-Ronen Y, Plotnikov A, Ching S, Stumpf MP, Klein OD, Seger R, Tzahor E. Nuclear to cytoplasmic shuttling of ERK promotes differentiation of muscle stem/progenitor cells. Development. 2014; 141:2611–2620. DOI: 10.1242/dev.107078. PMID: 24924195. PMCID: PMC4067960.
Article17. Han XH, Jin YR, Seto M, Yoon JK. A WNT/beta-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J Biol Chem. 2011; 286:10649–10659. DOI: 10.1074/jbc.M110.169391. PMID: 21252233. PMCID: PMC3060516.
Article18. Asfour HA, Allouh MZ, Said RS. Myogenic regulatory factors: the orchestrators of myogenesis after 30 years of discovery. Exp Biol Med (Maywood). 2018; 243:118–128. DOI: 10.1177/1535370217749494. PMID: 29307280. PMCID: PMC5788151.
Article19. Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007; 179:1043–1057. DOI: 10.1083/jcb.200703036. PMID: 18056416. PMCID: PMC2099183.
Article20. Atherton P, Stutchbury B, Jethwa D, Ballestrem C. Mechanosensitive components of integrin adhesions: role of vinculin. Exp Cell Res. 2016; 343:21–27. DOI: 10.1016/j.yexcr.2015.11.017. PMID: 26607713. PMCID: PMC4856733.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in Skeletal Muscle Stem Cells for Duchenne Muscular Dystrophy Treatment
- Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study
- Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars
- Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media
- Synergistic Effect of Hydrogen and 5-Aza on Myogenic Differentiation through the p38 MAPK Signaling Pathway in Adipose-Derived Mesenchymal Stem Cells