Obstet Gynecol Sci.
2019 Nov;62(6):445-453. 10.5468/ogs.2019.62.6.445.
Endometrial thickness cut-off value by transvaginal ultrasonography for screening of endometrial pathology in premenopausal and postmenopausal women
- Affiliations
-
- 1Health Screening and Promotion Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. swhlee@amc.seoul.kr
- KMID: 2462123
- DOI: http://doi.org/10.5468/ogs.2019.62.6.445
Abstract
OBJECTIVE
To assess the clinical usefulness and diagnostic accuracy of ultrasonographic measurement of endometrial thickness (ET) in women with endometrial hyperplasia or cancer (EH+).
METHODS
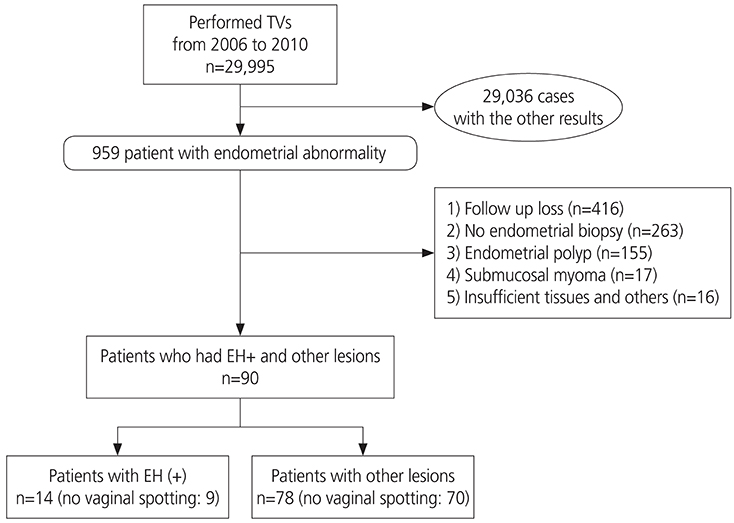
This retrospective cohort study included 29,995 consecutive women who underwent transvaginal ultrasonography (TVS) for an incidental finding of a thickened endometrium at the health screening and promotion center at Asan Medical Center between 2006 and 2010. Among 959 patients with endometrial abnormalities, 92 patients were included in this study. A total of 867 patients were excluded: 416 were lost to follow-up; 263 did not undergo endometrial biopsy; 155 had endometrial polyps; 17 had submucosal myomas; and 16 had insufficient tissue samples. Endometrial histology was the reference standard for calculating accuracy.
RESULTS
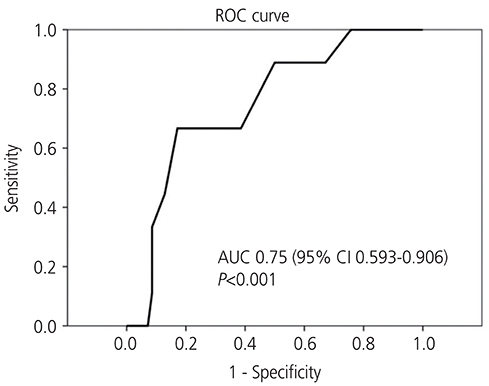
Of the 92 patients, 78 (84.8%) had normal pathology, while 14 (15.2%) had endometrial pathology (EH+), including 5 patients (35.7%) with simple hyperplasia without atypia, 3 (21.4%) with complex hyperplasia, and 6 (42.9%) with endometrial carcinoma, all stage Ia. The area under the receiver-operating characteristic curve was 0.75 (95% confidence interval [CI], 0.593-0.906). The cut-off value for ET was 8 mm, indicating that TVS ET had a fair accuracy in diagnosing carcinoma, had a sensitivity of 100% (95% CI, 62.9-100.0%) and a specificity of 24.3% (95% CI, 15.2-36.3%).
CONCLUSION
TVS is useful for detecting EH+, with a cut-off value for ET of 8 mm having a high sensitivity for detecting endometrial pathologies and the ability to identify women highly unlikely to have EH+, thereby avoiding more invasive endometrial biopsy.
MeSH Terms
Figure
Cited by 2 articles
-
Rate of premalignant and malignant endometrial lesion in “low-risk” premenopausal women with abnormal uterine bleeding undergoing endometrial biopsy
Sangam Jha, Akanksha Singh, Hemali Heidi Sinha, Poonam Bhadani, Monika Anant, Mukta Agarwal
Obstet Gynecol Sci. 2021;64(6):517-523. doi: 10.5468/ogs.21150.Endometrial thickness and uterine artery Doppler parameters as soft markers for prediction of endometrial cancer in postmenopausal bleeding women: a cross-sectional study at tertiary referral hospitals from Vietnam
Phuc Nhon Nguyen, Van Tuan Nguyen
Obstet Gynecol Sci. 2022;65(5):430-440. doi: 10.5468/ogs.22053.
Reference
-
1. von Gruenigen VE, Gil KM, Frasure HE, Jenison EL, Hopkins MP. The impact of obesity and age on quality of life in gynecologic surgery. Am J Obstet Gynecol. 2005; 193:1369–1375.
Article2. Bray F, Dos Santos Silva I, Moller H, Weiderpass E. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev. 2005; 14:1132–1142.
Article3. Haslam DW, James WP. Obesity. Lancet. 2005; 366:1197–1209.
Article4. Malkasian GD Jr, Annegers JF, Fountain KS. Carcinoma of the endometrium: stage I. Am J Obstet Gynecol. 1980; 136:872–888.
Article5. Goldstein SR, Zeltser I, Horan CK, Snyder JR, Schwartz LB. Ultrasonography-based triage for perimenopausal patients with abnormal uterine bleeding. Am J Obstet Gynecol. 1997; 177:102–108.
Article6. Astrup K, Olivarius NF, Møller S, Gottschau A, Karlslund W. Menstrual bleeding patterns in pre- and perimenopausal women: a population-based prospective diary study. Acta Obstet Gynecol Scand. 2004; 83:197–202.
Article7. Hallberg L, Högdahl AM, Nilsson L, Rybo G. Menstrual blood loss--a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966; 45:320–351.
Article8. Fleischer AC, Wheeler JE, Lindsay I, Hendrix SL, Grabill S, Kravitz B, et al. An assessment of the value of ultrasonographic screening for endometrial disease in postmenopausal women without symptoms. Am J Obstet Gynecol. 2001; 184:70–75.
Article9. Karlsson B, Granberg S, Wikland M, Ylöstalo P, Torvid K, Marsal K, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding--a Nordic multicenter study. Am J Obstet Gynecol. 1995; 172:1488–1494.
Article10. Gull B, Karlsson B, Milsom I, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003; 188:401–408.
Article11. Tsuda H, Nakamura H, Inoue T, Kawamura N, Adachi K, Bandera CA. Transvaginal ultrasonography of the endometrium in postmenopausal Japanese women. Gynecol Obstet Invest. 2005; 60:218–223.
Article12. Timmermans A, Opmeer BC, Khan KS, Bachmann LM, Epstein E, Clark TJ, et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2010; 116:160–167.13. Saidi MH, Sadler RK, Theis VD, Akright BD, Farhart SA, Villanueva GR. Comparison of sonography, sonohysterography, and hysteroscopy for evaluation of abnormal uterine bleeding. J Ultrasound Med. 1997; 16:587–591.
Article14. Smith RA, Manassaram-Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015; 65:30–54.
Article15. Lynch HT, Lynch JF. Lynch syndrome: history and current status. Dis Markers. 2004; 20:181–198.
Article16. Goldstein SR. Modern evaluation of the endometrium. Obstet Gynecol. 2010; 116:168–176.
Article17. van Hanegem N, Breijer MC, Khan KS, Clark TJ, Burger MP, Mol BW, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011; 68:155–164.
Article18. Murphy KT, Rotmensch J, Yamada SD, Mundt AJ. Outcome and patterns of failure in pathologic stages I-IV clear-cell carcinoma of the endometrium: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2003; 55:1272–1276.
Article19. Wong AS, Lao TT, Cheung CW, Yeung SW, Fan HL, Ng PS, et al. Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: a retrospective cohort study. BJOG. 2016; 123:439–446.
Article20. Kasraeian M, Asadi N, Ghaffarpasand F, Karimi AA. Value of transvaginal ultrasonography in endometrial evaluation of non-bleeding postmenopausal women. Climacteric. 2011; 14:126–131.
Article21. Jacobs I, Gentry-Maharaj A, Burnell M, Manchanda R, Singh N, Sharma A, et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: a case-control study within the UKCTOCS cohort. Lancet Oncol. 2011; 12:38–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of Vaginal Sonography in Evaluation of Endometrium in Postmenopausal Women
- Endometrial evaluation with transvaginal ultrasonography for the screening of endometrial hyperplasia or cancer in premenopausal and perimenopausal women
- Correlation of Progesterone Challenge Test and Endometrial Thickness in Postmenopausal Women
- The Change of Endometrial Thickness in Tamoxifen-treated Postmenopausal Breast Cancer Patients
- Endometrial polyp surveillance in premenopausal breast cancer patients using tamoxifen