Obstet Gynecol Sci.
2019 Nov;62(6):382-390. 10.5468/ogs.2019.62.6.382.
Consequences of chemotherapeutic agents on primordial follicles and future clinical applications
- Affiliations
-
- 1Olson Center for Women's Health, Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, NE, USA. soyoun.kim@unmc.edu
- 2Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea.
- 3VA Nebraska Western Iowa Health Care System, Omaha, NE, USA.
- KMID: 2462115
- DOI: http://doi.org/10.5468/ogs.2019.62.6.382
Abstract
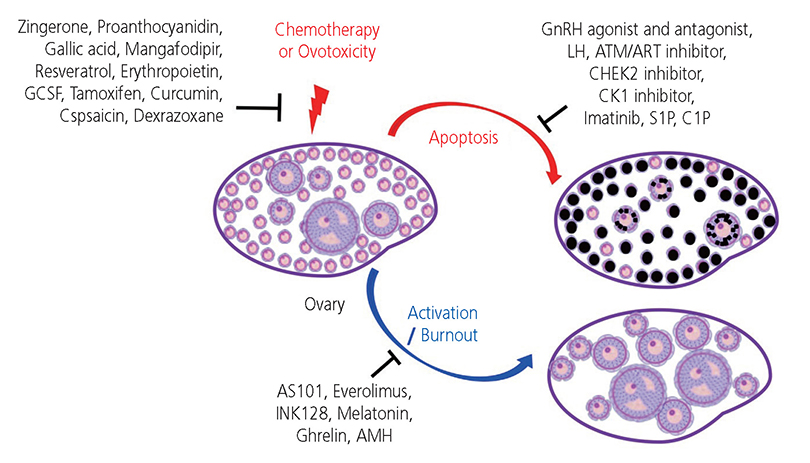
- The ovarian reserve is necessary for female fertility and endocrine health. Commonly used cancer therapies diminish the ovarian reserve, thus, resulting in primary ovarian insufficiency, which clinically presents as infertility and endocrine dysfunction. Prepubertal children who have undergone cancer therapies often experience delayed puberty or cannot initiate puberty and require endocrine support to maintain a normal life. Thus, developing an effective intervention to prevent loss of the ovarian reserve is an unmet need for these cancer patients. The selection of adjuvant therapies to protect the ovarian reserve against cancer therapies underlies the mechanism of loss of primordial follicles (PFs). Several theories have been proposed to explain the loss of PFs. The "burn out" theory postulates that chemotherapeutic agents activate dormant PFs through an activation pathway. Another theory posits that chemotherapeutic agents destroy PFs through an "apoptotic pathway" due to high sensitivity to DNA damage. However, the mechanisms causing loss of the ovarian reserve remains largely speculative. Here, we review current literature in this area and consider the mechanisms of how gonadotoxic therapies deplete PFs in the ovarian reserve.
MeSH Terms
Figure
Reference
-
1. Wilkosz P, Greggains GD, Tanbo TG, Fedorcsak P. Female reproductive decline is determined by remaining ovarian reserve and age. PLoS One. 2014; 9:e108343.
Article2. Findlay JK, Hutt KJ, Hickey M, Anderson RA. How is the number of primordial follicles in the ovarian reserve established? Biol Reprod. 2015; 93:111.
Article3. Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996; 8:485–489.
Article4. Sen A, Caiazza F. Oocyte maturation: a story of arrest and release. Front Biosci (Schol Ed). 2013; 5:451–477.
Article5. Picton HM. Activation of follicle development: the primordial follicle. Theriogenology. 2001; 55:1193–1210.
Article6. Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012; 143:139–149.
Article7. Rose SR, Horne VE, Howell J, Lawson SA, Rutter MM, Trotman GE, et al. Late endocrine effects of childhood cancer. Nat Rev Endocrinol. 2016; 12:319–336.
Article8. Chemaitilly W, Sklar CA. Childhood cancer treatments and associated endocrine late effects: a concise guide for the pediatric endocrinologist. Horm Res Paediatr. 2019; 91:74–82.
Article9. Livinalli A, Silva MT, Lopes LC. Late adverse effects related to treatment in a cohort of survivors of childhood and adolescent cancer. Medicine (Baltimore). 2019; 98:e14921.
Article10. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016; 12:2333–2344.
Article11. Madanat-Harjuoja LM, Malila N, Lähteenmäki P, Pukkala E, Mulvihill JJ, Boice JD Jr, et al. Risk of cancer among children of cancer patients - a nationwide study in Finland. Int J Cancer. 2010; 126:1196–1205.12. Kim SY, Kim SK, Lee JR, Woodruff TK. Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women. J Gynecol Oncol. 2016; 27:e22.
Article13. Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009; 360:902–911.
Article14. Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006; 444:624–628.
Article15. Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 2008; 135:3–12.
Article16. Kim SY, Cordeiro MH, Serna VA, Ebbert K, Butler LM, Sinha S, et al. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013; 20:987–997.
Article17. Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of PUMA and NOXA. Mol Cell. 2012; 48:343–352.
Article18. Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018; 25:261–269.
Article19. Rinaldi VD, Hsieh K, Munroe R, Bolcun-Filas E, Schimenti JC. Pharmacological inhibition of the DNA damage checkpoint prevents radiation-induced oocyte death. Genetics. 2017; 206:1823–1828.
Article20. Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014; 343:533–536.
Article21. Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, et al. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ. 2019; 26:502–515.
Article22. Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, et al. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol. 2006; 299:1–11.
Article23. Jagarlamudi K, Liu L, Adhikari D, Reddy P, Idahl A, Ottander U, et al. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One. 2009; 4:e6186.24. Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012; 356:24–30.
Article25. Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013; 12:3245–3246.
Article26. Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013; 5:185ra62.
Article27. Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, et al. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci U S A. 2017; 114:3186–3191.
Article28. Lande Y, Fisch B, Tsur A, Farhi J, Prag-Rosenberg R, Ben-Haroush A, et al. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod Biomed Online. 2017; 34:104–114.29. Jang H, Na Y, Hong K, Lee S, Moon S, Cho M, et al. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to the p27Kip1 promoter in primordial follicles. J Pineal Res. 2017; 63:e12432.30. John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008; 321:197–204.
Article31. Kano M, Sosulski AE, Zhang L, Saatcioglu HD, Wang D, Nagykery N, et al. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci U S A. 2017; 114:E1688–97.
Article32. Pépin D, Sabatini ME, Donahoe PK. Müllerian inhibiting substance/anti-Müllerian hormone as a fertility preservation agent. Curr Opin Endocrinol Diabetes Obes. 2018; 25:399–405.
Article33. Xu J, Xu F, Letaw JH, Park BS, Searles RP, Ferguson BM. Anti-Müllerian hormone is produced heterogeneously in primate preantral follicles and is a potential biomarker for follicle growth and oocyte maturation in vitro. J Assist Reprod Genet. 2016; 33:1665–1675.
Article34. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014; 20:370–385.
Article35. Josso N, Picard JY, Rey R, di Clemente N. Testicular anti-Müllerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev. 2006; 3:347–358.36. Josso N, Legeai L, Forest MG, Chaussain JL, Brauner R. An enzyme linked immunoassay for anti-Müllerian hormone: a new tool for the evaluation of testicular function in infants and children. J Clin Endocrinol Metab. 1990; 70:23–27.
Article37. Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, Pepinsky RB, et al. An immunoassay to detect human Müllerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab. 1990; 70:16–22.38. Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of anti-Müllerian hormone (AMH) on ovarian primordial follicle assembly. PLoS One. 2011; 6:e20087.
Article39. Yang MY, Cushman RA, Fortune JE. Anti-Müllerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol Hum Reprod. 2017; 23:282–291.40. Leonard RCF, Adamson DJA, Bertelli G, Mansi J, Yellowlees A, Dunlop J, et al. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol. 2017; 28:1811–1816.
Article41. Munhoz RR, Pereira AA, Sasse AD, Hoff PM, Traina TA, Hudis CA, et al. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016; 2:65–73.42. Meirow D, Assad G, Dor J, Rabinovici J. The GnRH antagonist cetrorelix reduces cyclophosphamide-induced ovarian follicular destruction in mice. Hum Reprod. 2004; 19:1294–1299.
Article43. Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995; 52:365–372.44. Lambertini M, Ceppi M, Poggio F, Peccatori FA, Azim HA Jr, Ugolini D, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015; 26:2408–2419.
Article45. Horicks F, Van Den Steen G, Gervy C, Clarke HJ, Demeestere I. Both in vivo FSH depletion and follicular exposure to Gonadotrophin-releasing hormone analogues in vitro are not effective to prevent follicular depletion during chemotherapy in mice. Mol Hum Reprod. 2018; 24:221–232.46. Rossi V, Lispi M, Longobardi S, Mattei M, Di Rella F, Salustri A, et al. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death Differ. 2017; 24:72–82.
Article47. Luan Y, Edmonds ME, Woodruff TK, Kim SY. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol. 2019; 240:243–256.
Article48. Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009; 15:1179–1185.
Article49. Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997; 3:1228–1232.
Article50. Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008; 10:1375–1403.
Article51. Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995; 136:242–252.
Article52. Pascuali N, Scotti L, Di Pietro M, Oubiña G, Bas D, May M, et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum Reprod. 2018; 33:844–859.
Article53. Kaygusuzoglu E, Caglayan C, Kandemir FM, Yıldırım S, Kucukler S, Kılınc MA, et al. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomed Pharmacother. 2018; 102:517–530.
Article54. Zhang JQ, Xing BS, Zhu CC, Shen M, Yu FX, Liu HL. Protective effect of proanthocyanidin against oxidative ovarian damage induced by 3-nitropropionic acid in mice. Genet Mol Res. 2015; 14:2484–2494.
Article55. Li B, Weng Q, Liu Z, Shen M, Zhang J, Wu W, et al. Selection of antioxidants against ovarian oxidative stress in mouse model. J Biochem Mol Toxicol. 2017; 31:e21997.
Article56. Qin Y, Iwase A, Murase T, Bayasula , Ishida C, Kato N, et al. Protective effects of mangafodipir against chemotherapy-induced ovarian damage in mice. Reprod Biol Endocrinol. 2018; 16:106.
Article57. Banu SK, Stanley JA, Sivakumar KK, Arosh JA, Burghardt RC. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol Appl Pharmacol. 2016; 303:65–78.
Article58. Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res. 2018; 11:33.
Article59. Li XH, Wang HP, Tan J, Wu YD, Yang M, Mao CZ, et al. Loss of pigment epithelium-derived factor leads to ovarian oxidative damage accompanied by diminished ovarian reserve in mice. Life Sci. 2019; 216:129–139.
Article60. Luderer U. Ovarian toxicity from reactive oxygen species. Vitam Horm. 2014; 94:99–127.
Article61. Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000; 6:1109–1114.62. Dayangan Sayan C, Tulmac OB, Karaca G, Ozkan ZS, Yalcin S, Devrim T, et al. Could erythropoietin reduce the ovarian damage of cisplatin in female rats? Gynecol Endocrinol. 2018; 34:309–313.
Article63. Akdemir A, Zeybek B, Akman L, Ergenoglu AM, Yeniel AO, Erbas O, et al. Granulocyte-colony stimulating factor decreases the extent of ovarian damage caused by cisplatin in an experimental rat model. J Gynecol Oncol. 2014; 25:328–333.
Article64. Ting AY, Petroff BK. Tamoxifen decreases ovarian follicular loss from experimental toxicant DMBA and chemotherapy agents cyclophosphamide and doxorubicin in the rat. J Assist Reprod Genet. 2010; 27:591–597.
Article65. Kropp J, Roti Roti EC, Ringelstetter A, Khatib H, Abbott DH, Salih SM. Dexrazoxane diminishes doxorubicin-induced acute ovarian damage and preserves ovarian function and fecundity in mice. PLoS One. 2015; 10:e0142588.
Article66. Roti Roti EC, Salih SM. Dexrazoxane ameliorates doxorubicin-induced injury in mouse ovarian cells. Biol Reprod. 2012; 86:96.
Article67. Salih SM, Ringelstetter AK, Elsarrag MZ, Abbott DH, Roti EC. Dexrazoxane abrogates acute doxorubicin toxicity in marmoset ovary. Biol Reprod. 2015; 92:73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcription factors in the maintenance and survival of primordial follicles
- Primordial follicle activation as new treatment for primary ovarian insufficiency
- Control of ovarian primordial follicle activation
- Preservation of ovarian follicle by concomitant administration of GnRH agonist I, II or GnRH antagonist during Cyclophosphamide or Paclitaxel chemotherapy in mice
- Cryopreservation of the Human Adult Ovarian Cortical Tissues by Vitrification