Korean J Radiol.
2016 Oct;17(5):771-778. 10.3348/kjr.2016.17.5.771.
Optimized Performance of FlightPlan during Chemoembolization for Hepatocellular Carcinoma: Importance of the Proportion of Segmented Tumor Area
- Affiliations
-
- 1Department of Radiology, Research Institute of Radiological Science, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul 06273, Korea. doctorlkh@yuhs.ac
- KMID: 2458070
- DOI: http://doi.org/10.3348/kjr.2016.17.5.771
Abstract
OBJECTIVE
To evaluate retrospectively the clinical effectiveness of FlightPlan for Liver (FPFL), an automated tumor-feeding artery detection software in cone-beam CT angiography (CBCTA), in identifying tumor-feeding arteries for the treatment of hepatocellular carcinoma (HCC) using three different segmentation sensitivities.
MATERIALS AND METHODS
The study included 50 patients with 80 HCC nodules who received transarterial chemoembolization. Standard digital subtracted angiography (DSA) and CBCTA were systematically performed and analyzed. Three settings of the FPFL software for vascular tree segmentation were tested for each tumor: the default, Group D; adjusting the proportion of segmented tumor area between 30 to 50%, Group L; and between 50 to 80%, Group H.
RESULTS
In total, 109 feeder vessels supplying 80 HCC nodules were identified. The negative predictive value of DSA, FPFL in groups D, L, and H was 56.8%, 87.7%, 94.2%, 98.5%, respectively. The accuracy of DSA, FPFL in groups D, L, and H was 62.6%, 86.8%, 93.4%, 95.6%, respectively. The sensitivity, negative predictive value (NPV), and accuracy of FPFL were higher in Group H than in Group D (p = 0.041, 0.034, 0.005). All three segmentation sensitivity groups showed higher specificity, positive predictive value, NPV, and accuracy of FPFL, as compared to DSA.
CONCLUSION
FlightPlan for Liver is a valuable tool for increasing detection of HCC tumor feeding vessels, as compared to standard DSA analysis, particularly in small HCC. Manual adjustment of segmentation sensitivity improves the accuracy of FPFL.
Keyword
MeSH Terms
-
Adult
Aged
Angiography, Digital Subtraction/methods
Carcinoma, Hepatocellular/blood supply/diagnostic imaging/pathology/*therapy
Chemoembolization, Therapeutic/*methods
Cone-Beam Computed Tomography/methods
Female
Humans
Liver Neoplasms/blood supply/diagnostic imaging/pathology/*therapy
Male
Middle Aged
Neovascularization, Pathologic/diagnostic imaging/therapy
Predictive Value of Tests
Retrospective Studies
Sensitivity and Specificity
Software
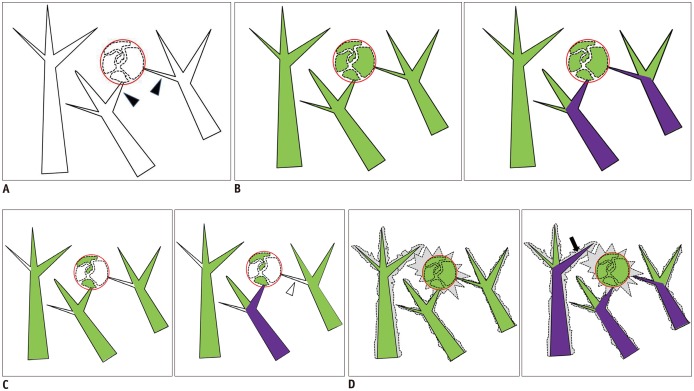
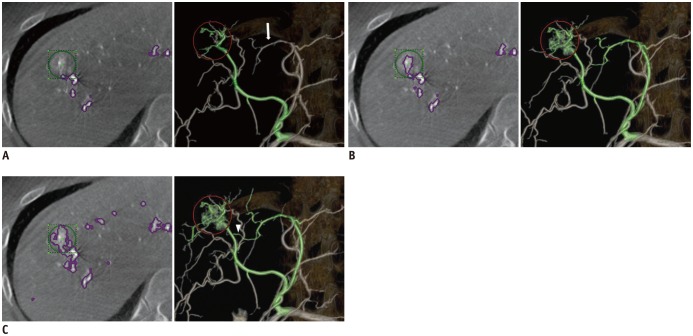
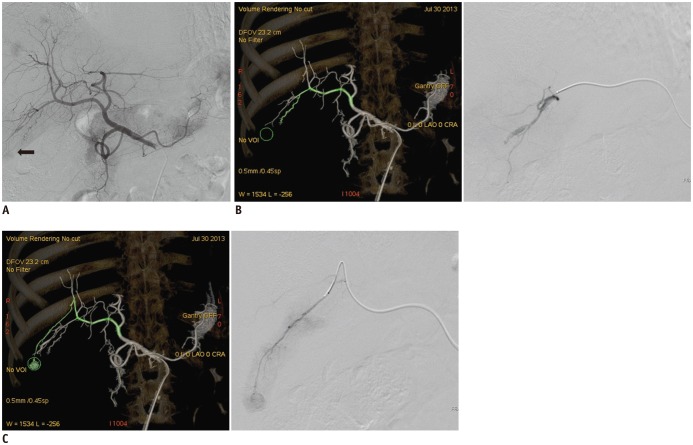
Figure
Reference
-
1. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–452. PMID: 25995680.2. Liu YS, Ou MC, Tsai YS, Lin XZ, Wang CK, Tsai HM, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015; 16:125–132. PMID: 25598680.
Article3. Han K, Kim JH, Yoon HM, Kim EJ, Gwon DI, Ko GY, et al. Transcatheter arterial chemoembolization for infiltrative hepatocellular carcinoma: clinical safety and efficacy and factors influencing patient survival. Korean J Radiol. 2014; 15:464–471. PMID: 25053906.
Article4. Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007; 18:365–376. PMID: 17377182.
Article5. Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011; 53:1580–1589. PMID: 21351114.6. Wallace MJ, Murthy R, Kamat PP, Moore T, Rao SH, Ensor J, et al. Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol. 2007; 18:1500–1507. PMID: 18057284.
Article7. Kim HC. Role of C-arm cone-beam CT in chemoembolization for hepatocellular carcinoma. Korean J Radiol. 2015; 16:114–124. PMID: 25598679.
Article8. Iwazawa J, Ohue S, Mitani T, Abe H, Hashimoto N, Hamuro M, et al. Identifying feeding arteries during TACE of hepatic tumors: comparison of C-arm CT and digital subtraction angiography. AJR Am J Roentgenol. 2009; 192:1057–1063. PMID: 19304714.
Article9. Deschamps F, Solomon SB, Thornton RH, Rao P, Hakime A, Kuoch V, et al. Computed analysis of three-dimensional cone-beam computed tomography angiography for determination of tumor-feeding vessels during chemoembolization of liver tumor: a pilot study. Cardiovasc Intervent Radiol. 2010; 33:1235–1242. PMID: 20390271.
Article10. Iwazawa J, Ohue S, Hashimoto N, Mitani T. Accuracy of software-assisted detection of tumour feeders in transcatheter hepatic chemoembolization using three target definition protocols. Clin Radiol. 2014; 69:145–150. PMID: 24268514.
Article11. Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol. 2012; 81:3985–3992. PMID: 22959287.
Article12. Pichon E, Bekes G, Deschamps F, Solomon SB. Development and preliminary evaluation of software for planning selective liver embolizations from three-dimensional rotational fluoroscopy imaging. Int J CARS. 2008; 3:405–412.
Article13. Kakeda S, Korogi Y, Ohnari N, Moriya J, Oda N, Nishino K, et al. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J Vasc Interv Radiol. 2007; 18:1508–1516. PMID: 18057285.
Article14. Virmani S, Ryu RK, Sato KT, Lewandowski RJ, Kulik L, Mulcahy MF, et al. Effect of C-arm angiographic CT on transcatheter arterial chemoembolization of liver tumors. J Vasc Interv Radiol. 2007; 18:1305–1309. PMID: 17911523.
Article15. Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Sugimori N, Igarashi S, et al. Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc Intervent Radiol. 2009; 32:255–264. PMID: 19067043.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is scheduled second chemoembolization necessary for early stage hepatocellular carcinoma showing complete response after first chemoembolization?
- Common Bile Duct Obstruction Caused by Tumor Thrombus after Trans-arterial Chemoembolization in a Hepatocellular Carcinoma Patient
- Chemoembolization combined with radiofrequency ablation is the best option for the local treatment of early hepatocellular carcinoma?
- Rupture of hepatocellular carcinoma after transcatheter arterial chemoembolization: A case report
- Intraductal migration of necrotic hepatocellular carcinoma: A possible cause of obstructive cholangitis after chemoembolization