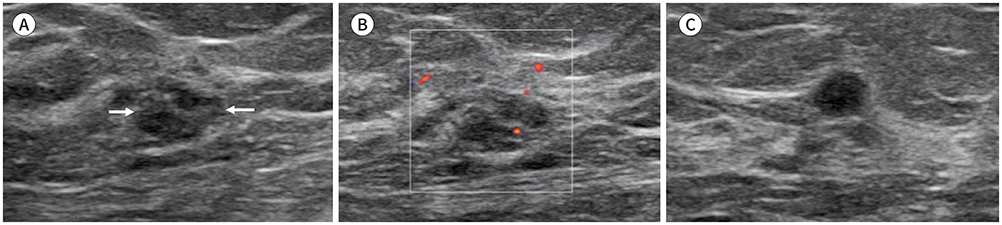
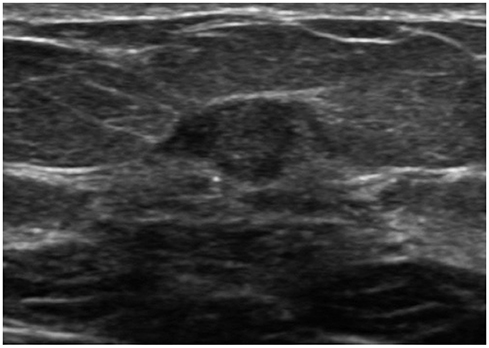
J Korean Soc Radiol.
2019 Jul;80(4):728-739. 10.3348/jksr.2019.80.4.728.
Small Breast Cancer (≤ 5 mm): Ultrasonographic Features and Clinical and Pathological Characteristics
- Affiliations
-
- 1Department of Radiology, Ewha Womans University Mokdong Hospital, Seoul, Korea. aqua0724@ewha.ac.kr
- KMID: 2457450
- DOI: http://doi.org/10.3348/jksr.2019.80.4.728
Abstract
- PURPOSE
To identify differences in ultrasonography (US) feature, clinical and pathological characteristics including immunohistochemical characteristics between small breast cancer (pathologic size ≤ 5 mm) and large breast cancer (> 5 mm).
MATERIALS AND METHODS
A total of 528 invasive breast cancer lesions in 475 patients were included. US features with clinical and pathological characteristics were evaluated according to pathologic size. US Breast Imaging-Reporting and Data System findings and final assessments were recorded for each lesion. Standard references were based on surgical pathologies.
RESULTS
Of 528 invasive breast cancer lesions, 62 were small breast cancers. Small breast cancers showed a higher rate of oval, round shape, parallel orientation; circumscribed margin; and iso/solid and cystic echo pattern, with no posterior feature. The final assessment of category 4 was also a dominant factor in small breast cancer. Early stage, asymptomatic state, and extensive ductal carcinoma in situ component were associated with small breast cancers.
CONCLUSION
Our results show that small breast cancers have less suspicious US features than large breast cancers.
MeSH Terms
Figure
Reference
-
1. American Cancer Society. Breast cancer prevention and early detection. Atlanta: American Cancer Society, Inc;2015.2. Moon HJ, Kim EK. Characteristics of breast cancer detected by supplementary screening ultrasonography. Ultrasonography. 2015; 34:153–156.
Article3. Wishart GC, Greenberg DC, Britton PD, Chou P, Brown CH, Purushotham AD, et al. Screen-detected vs symptomatic breast cancer: is improved survival due to stage migration alone? Br J Cancer. 2008; 98:1741–1744.
Article4. Karanlik H, Ozgur I, Sahin D, Fayda M, Onder S, Yavuz E. Intraoperative ultrasound reduces the need for reexcision in breast-conserving surgery. World J Surg Oncol. 2015; 13:321.
Article5. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717.6. Costantini M, Belli P, Bufi E, Asunis AM, Ferra E, Bitti GT. Association between sonographic appearances of breast cancers and their histopathologic features and biomarkers. J Clin Ultrasound. 2016; 44:26–33.
Article7. Torres-Tabanera M, Cárdenas-Rebollo JM, Villar-Castaño P, Sánchez-Gómez SM, Cobo-Soler J, Montoro-Martos EE, et al. Analysis of the positive predictive value of the subcategories of BI-RADS® 4 lesions: preliminary results in 880 lesions. Radiologia. 2012; 54:520–531.8. Hao SY, Ou B, Li LJ, Peng YL, Wang Y, Liu LS, et al. Could ultrasonic elastography help the diagnosis of breast cancer with the usage of sonographic BI-RADS classification? Eur J Radiol. 2015; 84:2492–2500.
Article9. Du HY, Lin BR, Huang DP. Ultrasonographic findings of triple-negative breast cancer. Int J Clin Exp Med. 2015; 15:10040–10043.10. Lee S, Jung Y, Bae Y. Synchronous BI-RADS category 3 lesions on preoperative ultrasonography in patients with breast cancer: is short-term follow-up appropriate? J Breast Cancer. 2015; 18:181–186.
Article11. Yoo EY, Shin JH, Ko EY, Han BK, Cho EY, Nam SJ, et al. Detectability and clinicohistological characteristics of small (≤ 1 cm) invasive breast cancer. Eur J Radiol. 2013; 82:e556–e561.12. Chen SC, Cheung YC, Su CH, Chen MF, Hwang TL, Hsueh S. Analysis of sonographic features for the differentiation of benign and malignant breast tumors of different sizes. Ultrasound Obstet Gynecol. 2004; 23:188–193.
Article13. Korpraphong P, Tritanon O, Tangcharoensathien W, Angsusinha T, Chuthapisith S. Ultrasonographic characteristics of mammographically occult small breast cancer. J Breast Cancer. 2012; 15:344–349.
Article14. American Joint Committee on Cancer (AJCC). AJCC cancer staging manual (part XI). 8th ed. Chicago: American College of Surgeons;2017. p. 599–600.15. American College of Radiology. ACR BI-RADS ultrasound. ACR breast imagingreporting and data system, breast imaging atlas. 5th ed. Reston: American College of Radiology;2013.16. Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990; 50:7057–7061.17. Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013; 139:539–552.
Article18. Aalders KC, Kuijer A, Straver ME, Slaets L, Litiere S, Viale G, et al. Characterisation of multifocal breast cancer using the 70-gene signature in clinical low-risk patients enrolled in the EORTC 10041/BIG 03-04 MINDACT trial. Eur J Cancer. 2017; 79:98–105.
Article19. Lynch SP, Lei X, Chavez-MacGregor M, Hsu L, Meric-Bernstam F, Buchholz TA, et al. Multifocality and multicentricity in breast cancer and survival outcomes. Ann Oncol. 2012; 23:3063–3069.
Article20. Gruber IV, Rueckert M, Kagan KO, Staebler A, Siegmann KC, Hartkopf A, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer. 2013; 13:328.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasonographic Characteristics of Mammographically Occult Small Breast Cancer
- Erratum to: Ultrasonographic Characteristics of Mammographically Occult Small Breast Cancer
- Ultrasonographic and Pathologic Findings of Dermatofibrosarcoma Protuberans of the Breast: A Case Report
- Ultrasonographic features of traumatic neuromas in breast cancer patients after mastectomy
- Clinical, Mammographic, and Ultrasonographic Assessment of Breast Cancer Size