J Menopausal Med.
2018 Dec;24(3):139-142. 10.6118/jmm.2018.24.3.139.
The Role of Menopausal Hormone Therapy in Reducing All-cause Mortality in Postmenopausal Women Younger than 60 Years: An Adaptive Meta-analysis
- Affiliations
-
- 1Department of Preventive Medicine, Jeju National University School of Medicine, Jeju, Korea.
- 2Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bkyoon@skku.edu
- KMID: 2455885
- DOI: http://doi.org/10.6118/jmm.2018.24.3.139
Abstract
- No abstract available.
MeSH Terms
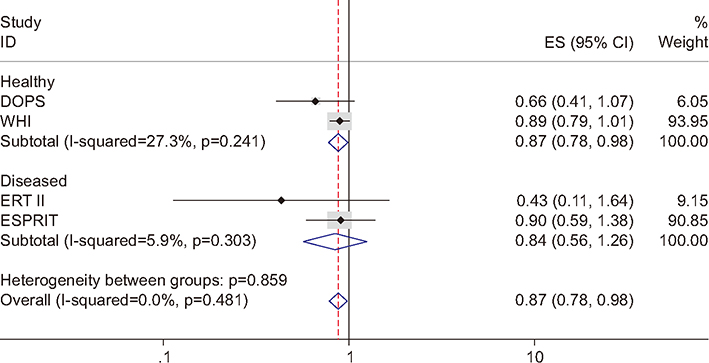
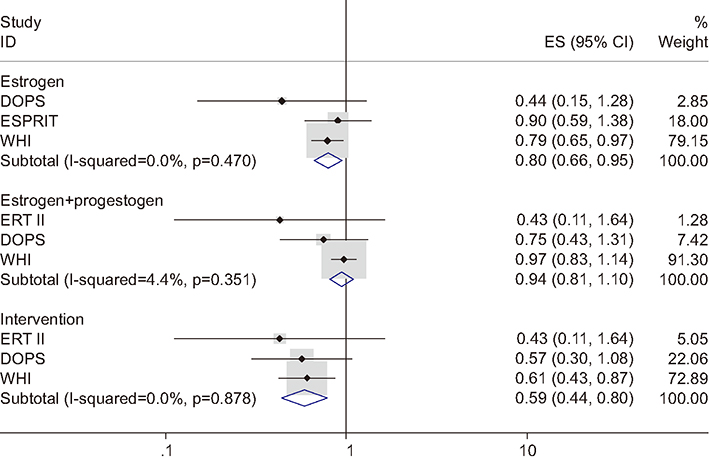
Figure
Cited by 1 articles
-
Risks and benefits of menopausal hormone therapy
Byung-Koo Yoon
J Korean Med Assoc. 2019;62(3):150-159. doi: 10.5124/jkma.2019.62.3.150.
Reference
-
1. Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010; 95:s1–s66.
Article2. Boardman HM, Hartley L, Eisinga A, Main C, Roqué i, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015; (3):CD002229.
Article3. Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012; 345:e6409.
Article4. Veerus P, Hovi SL, Fischer K, Rahu M, Hakama M, Hemminki E. Results from the estonian postmenopausal hormone therapy trial [ISRCTN35338757]. Maturitas. 2006; 55:162–173.
Article5. Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstet Gynecol. 1979; 54:74–79.
Article6. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002; 288:321–333.7. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004; 291:1701–1712.8. Gartlehner G, Patel SV, Viswanathan M, Feltner C, Weber RP, Lee R, et al. U.S. preventive services task force evidence syntheses, formerly systematic evidence reviews. Agency for Healthcare Research and Quality. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: An evidence review for the U.S. preventive services task force. Rockville (MD): Agency for Healthcare Research and Quality;2017. p. 15-05227-EF-1.9. LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011; 305:1305–1314.10. Grossman DC, Curry SJ, Owens DK, Barry MJ, Davidson KW, Doubeni CA, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US preventive services task force recommendation statement. JAMA. 2017; 318:2224–2233.11. Bae JM, Kim EH. Citation discovery tools for conducting adaptive meta-analyses to update systematic reviews. J Prev Med Public Health. 2016; 49:129–133.
Article12. Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The women’s health initiative randomized trials. JAMA. 2017; 318:927–938.13. Cherry N, McNamee R, Heagerty A, Kitchener H, Hannaford P. Long-term safety of unopposed estrogen used by women surviving myocardial infarction: 14-year follow-up of the ESPRIT randomised controlled trial. BJOG. 2014; 121:700–705.
Article14. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998; 280:605–613.
Article15. Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002; 288:58–66.
Article16. Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, et al. Effect of hormone therapy on mortality rates among women with heart failure and coronary artery disease. Am J Cardiol. 2005; 95:289–291.
Article17. Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Gastric Cancer. 2008; 11:23–32.
Article18. Shim SR, Shin IS, Bae JM. Intervention meta-analysis using STATA software. J Health Inform Stat. 2016; 41:123–134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mortality Associated with Hormone Replacement Therapy in Postmenopausal Women
- Awareness and Experience of Menopausal Symptom and Hormone Therapy in Korean Postmenopausal Women
- Updated treatment guideline for hormone therapy in postmenopausal women
- Skin Rejuvenation in Women using Menopausal Hormone Therapy: A Systematic Review and Meta-Analysis
- The effect of hormone replacement therapy on the postmenopausal symptoms In the women medicated continuously and the women quitted the medcation -