J Korean Med Assoc.
2019 Mar;62(3):145-149. 10.5124/jkma.2019.62.3.145.
Updated treatment guideline for hormone therapy in postmenopausal women
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Guro Hospital, Korea University College of Medicine, Seoul, Korea. shinjh@korea.ac.kr
- KMID: 2441065
- DOI: http://doi.org/10.5124/jkma.2019.62.3.145
Abstract
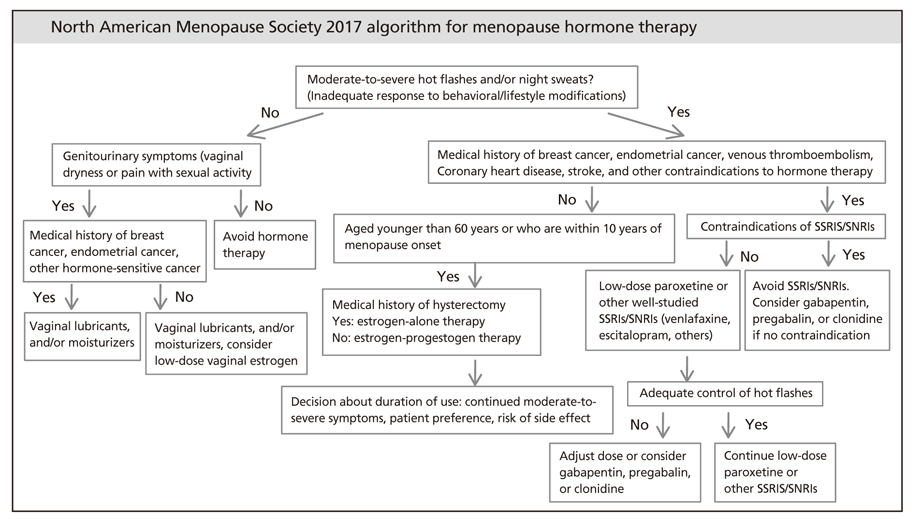
- Since menopause hormone therapy was first introduced, it has been widely used worldwide as the most effective treatment for vasomotor symptoms in menopausal women and for genitourinary syndrome of menopause. Menopause hormone therapy has been shown to prevent bone loss and fracture, but it may additionally offer various benefits for numerous other symptoms. The benefit-to-risk ratio of menopause hormone therapy is most favorable for women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications. Longer durations of therapy should be limited to patients with documented indications, such as persistent vasomotor symptoms or bone loss. For genitourinary syndrome of menopause, low-dose vaginal estrogen therapy or other therapies are recommended. Tibolone is a synthetic steroid that provides a therapeutic effect in the treatment of menopausal symptoms.
Keyword
Figure
Cited by 1 articles
-
Factors influencing quality of life in post-menopausal women
Hyunsook Shin, Eunjoo Lee
Korean J Women Health Nurs. 2020;26(4):336-345. doi: 10.4069/kjwhn.2020.11.14.
Reference
-
1. US Preventive Services Task Force. Grossman DC, Curry SJ, Owens DK, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017; 318:2224–2233.
Article2. Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015; 100:3975–4011.
Article3. Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004; (4):CD002978.
Article4. Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA. 2001; 285:2891–2897.
Article5. Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016; 106:1588–1599.
Article6. Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016; (8):CD001500.
Article7. Pinkerton JV, Abraham L, Bushmakin AG, Cappelleri JC, Racketa J, Shi H, Chines AA, Mirkin S. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt). 2014; 23:18–28.
Article8. Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, Liu J, McNeeley SG, Lopez AM. Women's Health Initiative Investigators. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA. 2003; 290:1739–1748.
Article9. Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause. 2004; 11:356–367.
Article10. Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006; 13:265–279.
Article11. Sarrel PM, Sullivan SD, Nelson LM. Hormone replacement therapy in young women with surgical primary ovarian insufficiency. Fertil Steril. 2016; 106:1580–1587.
Article12. Hong JS, Yi SW, Kang HC, Jee SH, Kang HG, Bayasgalan G, Ohrr H. Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas. 2007; 56:411–419.
Article13. Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, Chlebowski RT, Gass M, LaCroix A, Manson JE, Prentice RL, Rossouw J, Stefanick ML. WHI Investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008; 299:1036–1045.
Article14. Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017; 1:CD004143.
Article15. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013; 20:888–902.16. Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in postmenopausal women. Cochrane Database Syst Rev. 2012; 10:CD001405.
Article17. Joffe H, Petrillo LF, Koukopoulos A, Viguera AC, Hirschberg A, Nonacs R, Somley B, Pasciullo E, White DP, Hall JE, Cohen LS. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011; 96:E1044–E1054.
Article18. Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012; 19:697–703.
Article19. Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000; 183:414–420.
Article20. Barrett-Connor E, Wehren LE, Siris ES, Miller P, Chen YT, Abbott TA 3rd, Berger ML, Santora AC, Sherwood LM. Recency and duration of postmenopausal hormone therapy: effects on bone mineral density and fracture risk in the National Osteoporosis Risk Assessment (NORA) study. Menopause. 2003; 10:412–419.
Article21. Min YK, Lee DY, Choi SJ, Kim JH, Choi D, Yoon BK. Effects of adding alendronate to ongoing hormone therapy on bone mineral density in postmenopausal Korean women: a randomized, double-blind, placebo-controlled clinical trial. Menopause. 2013; 20:761–766.
Article22. Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, Kloosterboer L, Mosca L, Christiansen C, Bilezikian J, Kerzberg EM, Johnson S, Zanchetta J, Grobbee DE, Seifert W, Eastell R. LIFT Trial Investigators. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008; 359:697–708.
Article23. de Villiers TJ, Pines A, Panay N, Gambacciani M, Archer DF, Baber RJ, Davis SR, Gompel AA, Henderson VW, Langer R, Lobo RA, Plu-Bureau G, Sturdee DW. International Menopause Society. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2013; 16:316–337.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Hormone Replacement upon hs- CRP in Korean Postmenopausal Women
- The usefulness of laparoscopic myomectomy after Hormone Replacement Therapy in postmenopausal women with uterine myoma
- Breast Parenchymal Change on Mammography Following Postmenopausal Hormone Replacement Therapy
- A Survey of Obstetricians & Gynecologists' Attitudes on Hormone Replacement Therapy
- Summary of the 2023 Thai Menopause Society Clinical Practice Guideline on Menopausal Hormone Therapy