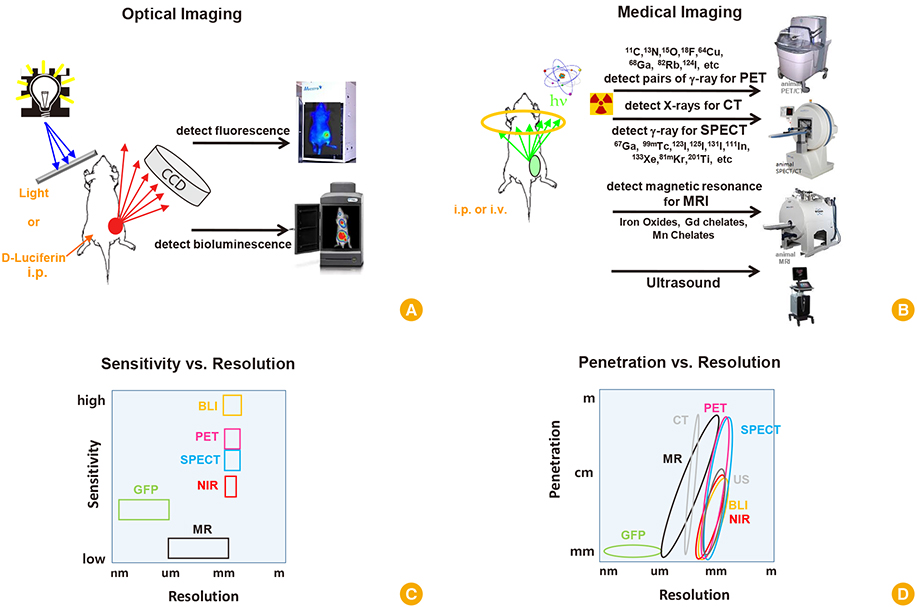
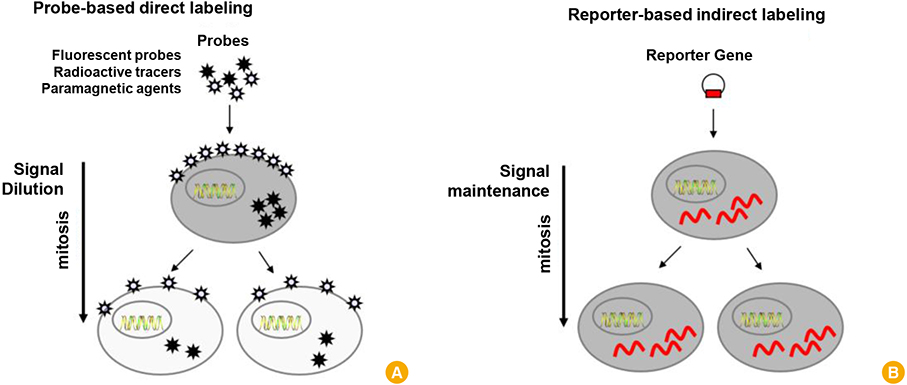
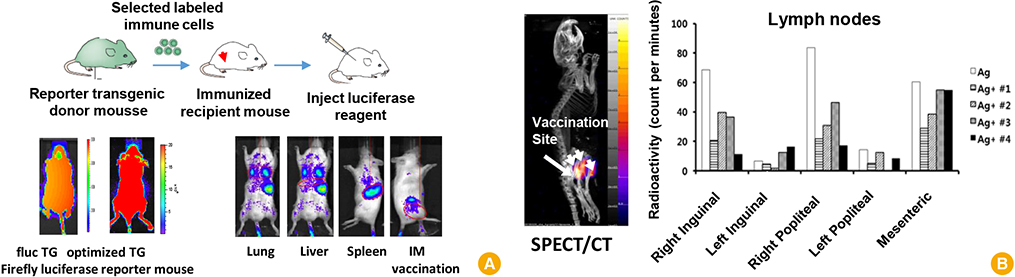
Clin Exp Vaccine Res.
2019 Jul;8(2):89-93. 10.7774/cevr.2019.8.2.89.
Non-invasive molecular imaging of immune cell dynamics for vaccine research
- Affiliations
-
- 1Department of Nuclear Medicine, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hwyoun@snu.ac.kr
- 2Cancer Imaging Center, Seoul National University Hospital, Seoul, Korea.
- 3G+flas Life Sciences, Seoul, Korea.
- KMID: 2455079
- DOI: http://doi.org/10.7774/cevr.2019.8.2.89
Abstract
- In order to develop a successful vaccine against deadly diseases with a wide range of antigenic diversity, an in-depth knowledge of the molecules and signaling mechanisms between the vaccine candidates and immune cells is required. Therefore, monitoring vaccine components, such as antigen or adjuvants, and immune cell dynamics at the vaccination site or draining lymph nodes can provide important information to understand more about the vaccine response. This review briefly introduces and describes various non-invasive molecular imaging methods for visualizing immune cell dynamics after vaccination.
Figure
Reference
-
1. Moxon ER, Siegrist CA. The next decade of vaccines: societal and scientific challenges. Lancet. 2011; 378:348–359.
Article2. Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006; 124:849–863.
Article3. Youn H, Hong KJ. In vivo non invasive molecular imaging for immune cell tracking in small animals. Immune Netw. 2012; 12:223–229.
Article4. Kinnear E, Caproni LJ, Tregoning JS. A comparison of red fluorescent proteins to model DNA vaccine expression by whole animal in vivo imaging. PLoS One. 2015; 10:e0130375.
Article5. Mandl S, Schimmelpfennig C, Edinger M, Negrin RS, Contag CH. Understanding immune cell trafficking patterns via in vivo bioluminescence imaging. J Cell Biochem Suppl. 2002; 39:239–248.
Article6. Rabinovich BA, Ye Y, Etto T, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci U S A. 2008; 105:14342–14346.
Article7. Preuss E, Treschow A, Newrzela S, et al. TK.007: A novel, codon-optimized HSVtk(A168H) mutant for suicide gene therapy. Hum Gene Ther. 2010; 21:929–941.
Article8. Kim YH, Youn H, Na J, et al. Codon-optimized human sodium iodide symporter (opt-hNIS) as a sensitive reporter and efficient therapeutic gene. Theranostics. 2015; 5:86–96.
Article9. Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev Immunol. 2006; 6:484–490.
Article10. Song MG, Kang B, Jeon JY, et al. In vivo imaging of differences in early donor cell proliferation in graft-versus-host disease hosts with different pre-conditioning doses. Mol Cells. 2012; 33:79–86.
Article11. Coates EE, Costner PJ, Nason MC, et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med. 2017; 42:329–334.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vivo Non Invasive Molecular Imaging for Immune Cell Tracking in Small Animals
- Imaging in Tumor Immunology
- AIDS Vaccine Development: the Past, the Present, and the Future
- Microbiota Influences Vaccine and Mucosal Adjuvant Efficacy
- Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease