Cancer Res Treat.
2019 Jul;51(3):841-850. 10.4143/crt.2019.151.
Autoimmune Diseases and Gastric Cancer Risk: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD, USA. minkyo.song@nih.gov
- 2Department of Internal Medicine, Pontifical Catholic University of Chile, Santiago, Chile.
- KMID: 2454277
- DOI: http://doi.org/10.4143/crt.2019.151
Abstract
- PURPOSE
Autoimmunity is an alternative etiology of gastric inflammation, the initiating event in the gastric carcinogenic cascade. This mechanism may be an increasingly important cause of gastric cancer with the waning prevalence of its primary etiologic factor, chronic Helicobacter pylori infection.
MATERIALS AND METHODS
PubMed and EMBASE were searched up to September 2018. Autoimmunity and 96 specific manifestations were considered for associations with gastric cancer risk. Random effects analysis was used to calculate pooled relative risk estimates (RR) and 95% confidence intervals (CI).
RESULTS
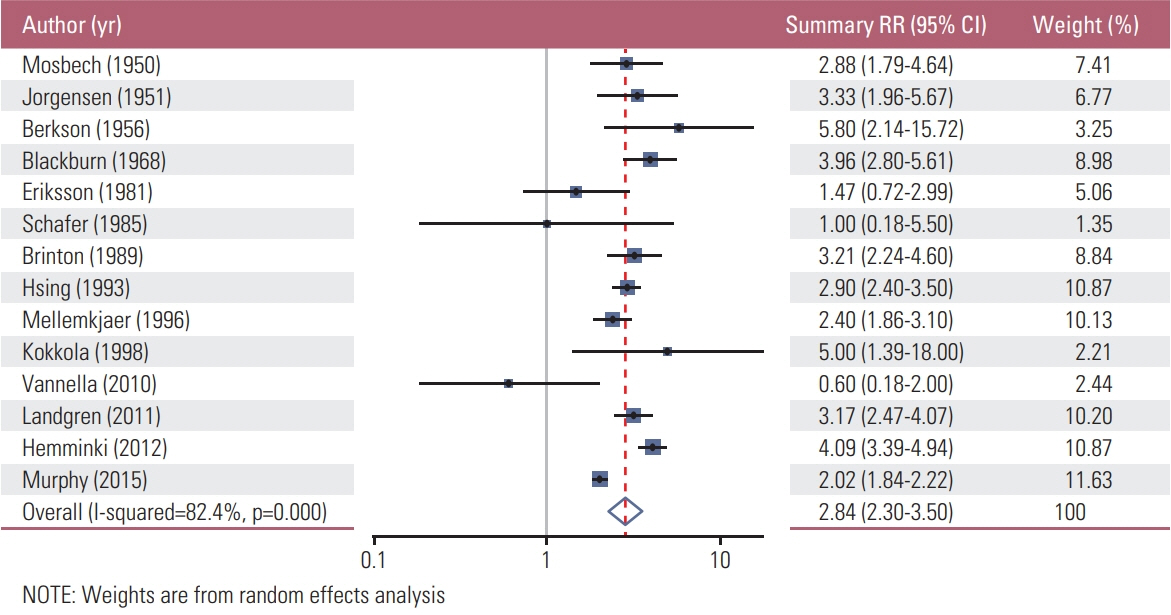
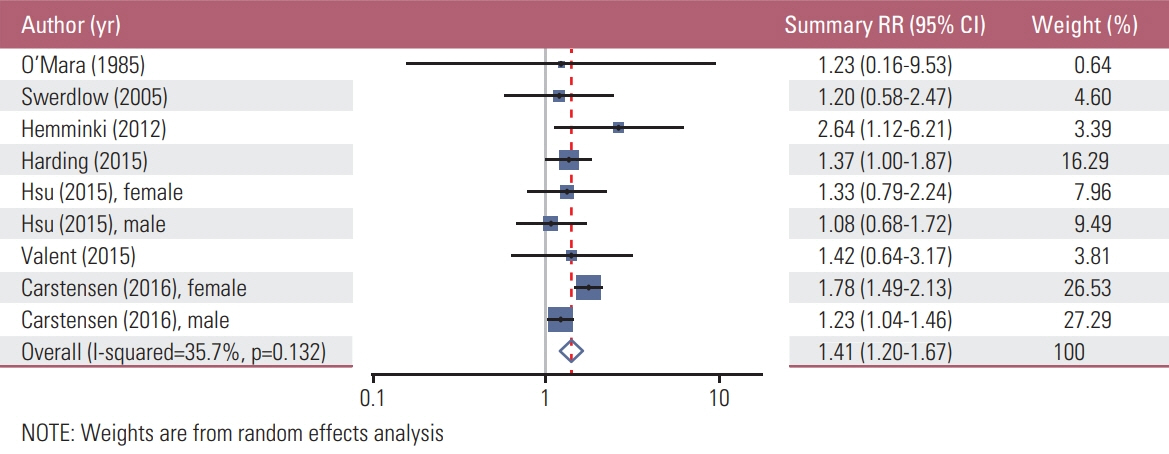
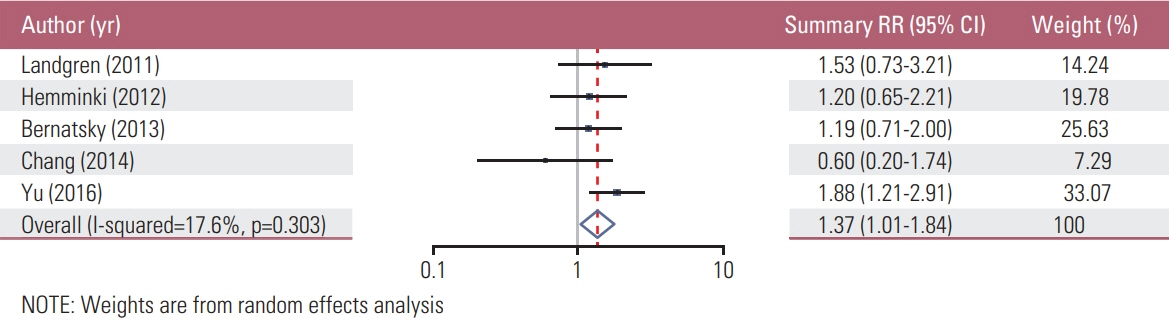
We found a total of 52 observational studies representing 30 different autoimmune diseases. Overall, the presence of an autoimmune condition was associated with a gastric cancer pooled RR of 1.37 (95% CI, 1.24 to 1.52). Among the 24 autoimmune conditions with two or more independent reports, nine were significantly associated with increased gastric cancer risk: dermatomyositis (RR, 3.69; 95% CI, 1.74 to 7.79), pernicious anemia (RR, 2.84; 95% CI, 2.30 to 3.50), Addison disease (RR, 2.11; 95% CI, 1.26 to 3.53), dermatitis herpetiformis (RR, 1.74; 95% CI, 1.02 to 2.97; n=3), IgG4-related disease (RR, 1.69; 95% CI, 1.00 to 2.87), primary biliary cirrhosis (RR, 1.64; 95% CI, 1.13 to 2.37), diabetes mellitus type 1 (RR, 1.41; 95% CI, 1.20 to 1.67), systemic lupus erythematosus (RR, 1.37; 95% CI, 1.01 to 1.84), and Graves disease (RR, 1.27; 95% CI, 1.06 to 1.52).
CONCLUSION
Our analysis documents the wide range of autoimmune diseases associated with gastric cancer. These associations may reflect unreported links between these conditions and autoimmune gastritis. Further studies are warranted to investigate potential causal mechanisms.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017; 153:420–9.
Article3. Anderson WF, Rabkin CS, Turner N, Fraumeni JF Jr, Rosenberg PS, Camargo MC. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst. 2018; 110:608–15.
Article4. Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: age, period and birth cohort analysis. Int J Cancer. 2017; 141:1333–44.
Article5. Eom BW, Jung KW, Won YJ, Yang H, Kim YW. Trends in gastric cancer incidence according to the clinicopathological characteristics in Korea, 1999-2014. Cancer Res Treat. 2018; 50:1343–50.
Article6. Song M, Rabkin CS, Camargo MC. Gastric cancer: an evolving disease. Curr Treat Options Gastroenterol. 2018; 16:561–9.
Article7. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002; 347:911–20.
Article8. Toh BH. Diagnosis and classification of autoimmune gastritis. Autoimmun Rev. 2014; 13:459–62.
Article9. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute;2018. [cited 2018 Oct 5]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62:e1–34.
Article11. Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Autoimmune disease and subsequent digestive tract cancer by histology. Ann Oncol. 2012; 23:927–33.
Article12. Landgren AM, Landgren O, Gridley G, Dores GM, Linet MS, Morton LM. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer. 2011; 117:1163–71.
Article13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.
Article14. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.
Article15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.
Article16. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–63.
Article17. Blackburn EK, Callender ST, Dacie JV, Doll R, Girdwood RH, Mollin DL, et al. Possible association between pernicious anaemia and leukaemia: a prospective study of 1,625 patients with a note on the very high incidence of stomach cancer. Int J Cancer. 1968; 3:163–70.
Article18. Brinton LA, Gridley G, Hrubec Z, Hoover R, Fraumeni JF Jr. Cancer risk following pernicious anaemia. Br J Cancer. 1989; 59:810–3.
Article19. Eriksson S, Clase L, Moquist-Olsson I. Pernicious anemia as a risk factor in gastric cancer. The extent of the problem. Acta Med Scand. 1981; 210:481–4.20. Hsing AW, Hansson LE, McLaughlin JK, Nyren O, Blot WJ, Ekbom A, et al. Pernicious anemia and subsequent cancer: a population-based cohort study. Cancer. 1993; 71:745–50.
Article21. Kokkola A, Sjoblom SM, Haapiainen R, Sipponen P, Puolakkainen P, Jarvinen H. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia: a prospective follow-up study. Scand J Gastroenterol. 1998; 33:88–92.22. Mellemkjaer L, Gridley G, Moller H, Hsing AW, Linet MS, Brinton LA, et al. Pernicious anaemia and cancer risk in Denmark. Br J Cancer. 1996; 73:998–1000.
Article23. Mosbech J, Videbaek A. Mortality from and risk of gastric carcinoma among patients with pernicious anaemia. Br Med J. 1950; 2:390–4.
Article24. Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A, et al. Cancer risk after pernicious anemia in the US elderly population. Clin Gastroenterol Hepatol. 2015; 13:2282–9. e1-4.
Article25. Schafer LW, Larson DE, Melton LJ 3rd, Higgins JA, Zinsmeister AR. Risk of development of gastric carcinoma in patients with pernicious anemia: a population-based study in Rochester, Minnesota. Mayo Clin Proc. 1985; 60:444–8.
Article26. Jorgensen J. The mortality among patients with pernicious anemia in Denmark and the incidence of gastric carcinoma among the same. Acta Med Scand. 1951; 139:472–81.27. Vannella L, Lahner E, Osborn J, Bordi C, Miglione M, Delle Fave G, et al. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment Pharmacol Ther. 2010; 31:1042–50.
Article28. Berkson J, Butt HR, Comfort MW. Occurrence of gastric cancer in persons with achlorhydria and with pernicious anemia. Proc Staff Meet Mayo Clin. 1956; 31:583–96.29. Carstensen B, Read SH, Friis S, Sund R, Keskimaki I, Svensson AM, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016; 59:980–8.
Article30. Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015; 38:264–70.
Article31. Valent F. Diabetes mellitus and cancer of the digestive organs: an Italian population-based cohort study. J Diabetes Complications. 2015; 29:1056–61.
Article32. Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005; 92:2070–5.
Article33. O'Mara BA, Byers T, Schoenfeld E. Diabetes mellitus and cancer risk: a multisite case-control study. J Chronic Dis. 1985; 38:435–41.34. Hsu PC, Lin WH, Kuo TH, Lee HM, Kuo C, Li CY. A population-based cohort study of all-cause and site-specific cancer incidence among patients with type 1 diabetes mellitus in Taiwan. J Epidemiol. 2015; 25:567–73.
Article35. Bernatsky S, Ramsey-Goldman R, Labrecque J, Joseph L, Boivin JF, Petri M, et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun. 2013; 42:130–5.
Article36. Chang SH, Park JK, Lee YJ, Yang JA, Lee EY, Song YW, et al. Comparison of cancer incidence among patients with rheumatic disease: a retrospective cohort study. Arthritis Res Ther. 2014; 16:428.
Article37. Yu KH, Kuo CF, Huang LH, Huang WK, See LC. Cancer risk in patients with inflammatory systemic autoimmune rheumatic diseases: a nationwide population-based dynamic cohort study in Taiwan. Medicine (Baltimore). 2016; 95:e3540.38. Dreyer L, Faurschou M, Mogensen M, Jacobsen S. High incidence of potentially virus-induced malignancies in systemic lupus erythematosus: a long-term followup study in a Danish cohort. Arthritis Rheum. 2011; 63:3032–7.
Article39. Seo HM, Han SS, Kim JS. Cancer risks among patients with alopecia areata: a population-based case-control study in Korea. J Am Acad Dermatol. 2018; 78:209–11.
Article40. Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr. 2004; 24:299–326.
Article41. Ji J, Sundquist J, Sundquist K. Family history of autoimmune diseases and risk of gastric cancer: a national cohort study. Eur J Cancer Prev. 2018; 27:221–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Helicobacter pylori Eradication and Risks of Metachronous Recurrence after Endoscopic Resection of Gastric Adenoma: A Systematic Review and Meta-Analysis
- Systematic Review and Meta-analysis in Digestive Cancer Research
- Association of Proton Pump Inhibitor Use With Gastric Cancer in Regions With High Prevalence of Gastric Cancer: Systematic Review and Meta-analysis
- Outcomes of Gastrectomy for Gastric Cancer in Patients Aged >80 Years: A Systematic Literature Review and Meta-Analysis
- An Introduction of the Systematic Review and Meta-Analysis