Korean J Gastroenterol.
2019 Jun;73(6):332-340. 10.4166/kjg.2019.73.6.332.
Nodular Gastritis as a Precursor Lesion of Atrophic and Metaplastic Gastritis
- Affiliations
-
- 1Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea. sunyoung@kuh.ac.kr
- KMID: 2454094
- DOI: http://doi.org/10.4166/kjg.2019.73.6.332
Abstract
- BACKGROUND/AIMS
Chronic atrophic gastritis (CAG) and metaplastic gastritis (MG) are precancerous conditions of Helicobacter pylori (H. pylori)-related gastric cancer. This study aimed to identify the characteristics of nodular gastritis (NG) showing CAG or MG after nodule regression.
METHODS
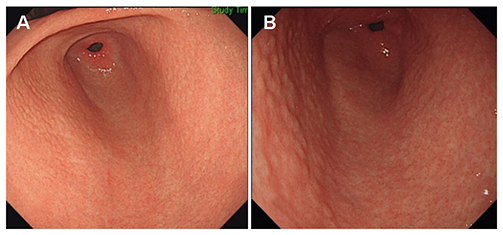
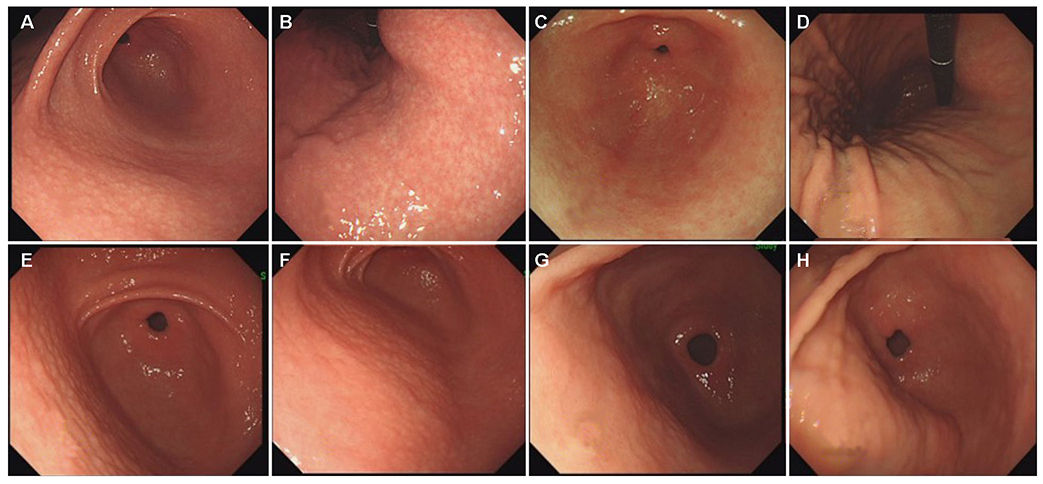
H. pylori-infected patients with NG were included after upper gastrointestinal endoscopy. Patients were excluded if their latest endoscopy had been performed ≤36 months after the initial diagnosis of NG. Small-granular-type NG was defined as the condition with 1-2 mm regular subepithelial nodules. Large-nodular-type NG was defined as those with 3-4 mm, irregular subepithelial nodules. The endoscopic findings after nodule regression were recorded.
RESULTS
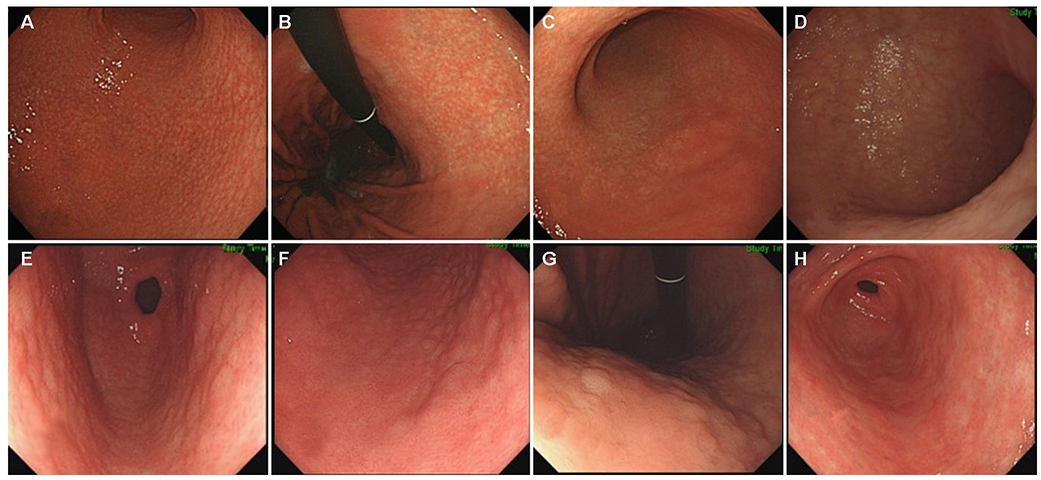
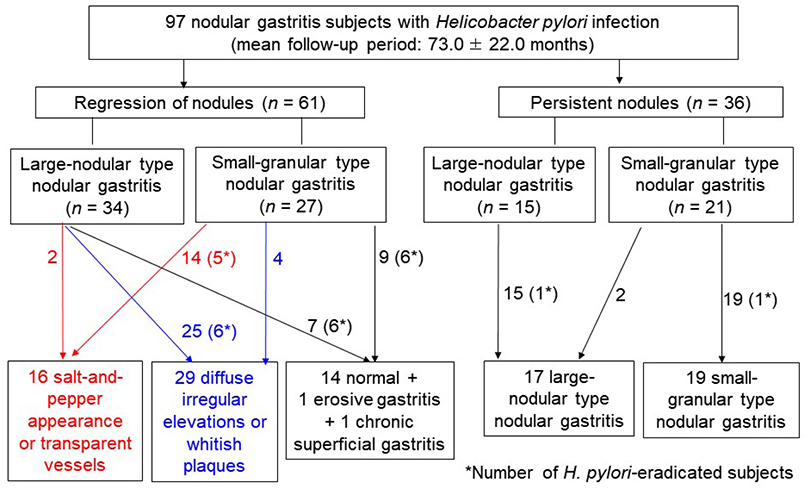
Among the 97 H. pylori-infected patients with NG, 61 showed nodule regression after a mean follow-up of 73.0±22.0 months. After nodule regression, 16 patients showed a salt-and-pepper appearance and/or transparent submucosal vessels, indicating CAG. Twenty-nine patients showed diffuse irregular elevations and/or whitish plaques, indicating MG. Sixteen patients with other endoscopic findings (14 normal, one erosive gastritis, and one chronic superficial gastritis) showed a higher proportion of H. pylori eradication (12/16, 75.0%) than those in the CAG group (5/16, 31.3%) and MG group (6/29, 20.7%; p=0.001). Patients with small-granular-type NG tended to progress toward CAG (14/27, 51.9%), whereas those with large-nodular-type NG tended to progress toward MG (25/34, 73.5%; p<0.001).
CONCLUSIONS
In patients with a persistent H. pylori infection, NG tended to progress to CAG or MG when the nodules regressed. Small-granular-type NG tended to progress to CAG, whereas large-nodular-type NG tended to progress to MG.
Keyword
MeSH Terms
Figure
Reference
-
1. Miyamoto M, Haruma K, Yoshihara M, et al. Nodular gastritis in adults is caused by Helicobacter pylori infection. Dig Dis Sci. 2003; 48:968–975.2. Chen MJ, Wang TE, Chang WH, Liao TC, Lin CC, Shih SC. Nodular gastritis: an endoscopic indicator of Helicobacter pylori infection. Dig Dis Sci. 2007; 52:2662–2666.3. Okamura T, Sakai Y, Hoshino H, Iwaya Y, Tanaka E, Kobayashi M. Superficially located enlarged lymphoid follicles characterise nodular gastritis. Pathology. 2015; 47:38–44.
Article4. Nakashima R, Nagata N, Watanabe K, et al. Histological features of nodular gastritis and its endoscopic classification. J Dig Dis. 2011; 12:436–442.5. Kim YJ, Lee SY, Lee SP, et al. Identification of nodular gastritis among patients diagnosed with lymphofollicular gastritis on gastric biopsied specimen. Korean J Gastroenterol. 2018; 71:143–152.6. Al-Enezi SA, Alsurayei SA, Aly NY, et al. Endoscopic nodular gastritis in dyspeptic adults: prevalence and association with Helicobacter pylori infection. Med Princ Pract. 2010; 19:40–45.7. Hong SN, Jo S, Jang JH, et al. Clinical characteristics and the expression profiles of inflammatory cytokines/cytokine regulatory factors in asymptomatic patients with nodular gastritis. Dig Dis Sci. 2012; 57:1486–1495.
Article8. Ebert EC, Hagspiel KD. Gastrointestinal and hepatic manifestations of rheumatoid arthritis. Dig Dis Sci. 2011; 56:295–302.9. Chen MJ, Shih SC, Wang TE, Chan YJ, Chen CJ, Chang WH. Endoscopic patterns and histopathological features after eradication therapy in Helicobacter pylori-associated nodular gastritis. Dig Dis Sci. 2008; 53:1893–1897.
Article10. Nishikawa I, Kato J, Terasoma S, et al. Nodular gastritis in association with gastric cancer development before and after Helicobacter pylori eradication. JGH Open. 2018; 2:80–86.11. Kim JW, Lee SY, Kim JH, et al. Nodule regression in adults with nodular gastritis. Gastroenterology Res. 2015; 8:296–302.
Article12. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014; 29:1371–1386.13. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969; 1:87–97.
Article14. Ahn SY, Lee SY, Hong SN, et al. Endoscopic diagnosis of open-type atrophic gastritis is related to the histological diagnosis of intestinal metaplasia and Cdx2 expression. Dig Dis Sci. 2011; 56:1119–1126.15. Nomura S, Ida K, Terao S, et al. Endoscopic diagnosis of gastric mucosal atrophy: multicenter prospective study. Dig Endosc. 2014; 26:709–719.
Article16. Fukuta N, Ida K, Kato T, et al. Endoscopic diagnosis of gastric intestinal metaplasia: a prospective multicenter study. Dig Endosc. 2013; 25:526–534.17. Sugimoto M, Ban H, Ichikawa H, et al. Efficacy of the Kyoto classification of gastritis in identifying patients at high risk for gastric cancer. Intern Med. 2017; 56:579–586.
Article18. Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017; 32:1581–1586.19. Niknam R, Manafi A, Maghbool M, Kouhpayeh A, Mahmoudi L. Is endoscopic nodular gastritis associated with premalignant lesions? Neth J Med. 2015; 73:236–241.20. Kitamura S, Yasuda M, Muguruma N, et al. Prevalence and characteristics of nodular gastritis in Japanese elderly. J Gastroenterol Hepatol. 2013; 28:1154–1160.21. Lee SP, Lee SY, Kim JH, Sung IK, Park HS, Shim CS. Link between serum pepsinogen concentrations and upper gastrointestinal endoscopic findings. J Korean Med Sci. 2017; 32:796–802.
Article22. Hayashi S, Imamura J, Kimura K, Saeki S, Hishima T. Endoscopic features of lymphoid follicles in Helicobacter pylori-associated chronic gastritis. Dig Endosc. 2015; 27:53–60.23. Kamada T, Sugiu K, Hata J, et al. Evaluation of endoscopic and histological findings in Helicobacter pylori-positive Japanese young adults. J Gastroenterol Hepatol. 2006; 21(1 Pt 2):258–261.24. Japan Helicobacter Society Guideline Committee. Guidelines for diagnosis and treatment of H. pylori infection. Tokyo: Sentan Igaku-sha;2016.25. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017; 112:212–239.26. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017; 66:6–30.27. Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016; 151:51–69.e14.28. Smith S, Boyle B, Brennan D, et al. The Irish Helicobacter pylori working group consensus for the diagnosis and treatment of H. pylori infection in adult patients in Ireland. Eur J Gastroenterol Hepatol. 2017; 29:552–559.
Article29. Sheu BS, Wu MS, Chiu CT, et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017; 22:e12368.30. Mahachai V, Vilaichone RK, Pittayanon R, et al. Helicobacter pylori management in ASEAN: the Bangkok consensus report. J Gastroenterol Hepatol. 2018; 33:37–56.31. International Agency for Research on Cancer, World Health Organization. Helicobacter pylori eradication as a strategy for preventing gastric cancer. IARC working group reports volume 8. Lyon: International Agency for Research on Cancer;2014.32. Shiotani A, Kamada T, Kumamoto M, et al. Nodular gastritis in Japanese young adults: endoscopic and histological observations. J Gastroenterol. 2007; 42:610–615.33. Sokmensuer C, Onal IK, Yeniova O, et al. What are the clinical implications of nodular gastritis? Clues from histopathology. Dig Dis Sci. 2009; 54:2150–2154.
Article34. Dwivedi M, Misra SP, Misra V. Nodular gastritis in adults: clinical features, endoscopic appearance, histopathological features, and response to therapy. J Gastroenterol Hepatol. 2008; 23:943–947.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histopathologic Diagnosis of Atrophic Gastritis and Intestinal Metaplasia
- Survey on the Endoscopic Diagnosis of Chronic Gastritis
- Atrophic Gastritis: Reversible after Treatment?
- Successful Eradication of Helicobacter pylori Using Modified Quadruple Therapy in Patient with Long-lasting H. pylori-induced Active Gastritis
- Atrophic Gastritis: Pathophysiology and Etiology