Korean Circ J.
2019 Jun;49(6):469-484. 10.4070/kcj.2019.0136.
Sacubitril/Valsartan in Asian Patients with Heart Failure with Reduced Ejection Fraction
- Affiliations
-
- 1British Heart Foundation Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK. john.mcmurray@glasgow.ac.uk
- KMID: 2454042
- DOI: http://doi.org/10.4070/kcj.2019.0136
Abstract
- The Prospective comparison of Angiotensin Receptor-neprilysin inhibitor (ARNI) with Angiotensin converting enzyme inhibitor (ACEI) to Determine Impact on Global Mortality and morbidity in Heart Failure (HF) trial (PARADIGM-HF) showed that adding a neprilysin inhibitor (sacubitril) to a renin-angiotensin system blocker (and other standard therapy) reduced morbidity and mortality in ambulatory patients with chronic HF with reduced ejection fraction (HFrEF). In PARADIGM-HF, valsartan combined with sacubitril (a so-called ARNI) was superior to the current gold standard of an ACEI, specifically enalapril, reducing the risk of the primary composite outcome of cardiovascular (CV) death or first HF hospitalization by 20% and all-cause death by 16%. Following the results of PARADIGM-HF, sacubitril/valsartan was approved by American and European regulatory authorities for the treatment of HFrEF. The burden of HF in Asia is substantial, both due to the huge population of the region and as a result of increasing CV risk factors and disease. Both the prevalence and mortality associated with HF are high in Asia. In the following review, we discuss the development of sacubitril/valsartan, the prototype ARNI, and the available evidence for its efficacy and safety in Asian patients with HFrEF.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Economic Burden of Heart Failure in Asian Countries Based on Real-world Data
Hyemoon Chung, Il Suk Sohn
Korean Circ J. 2021;51(8):694-695. doi: 10.4070/kcj.2021.0197.
Reference
-
1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017; 3:7–11.
Article2. Lam CS, Teng TK, Tay WT, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016; 37:3141–3153.
Article3. Dokainish H, Teo K, Zhu J, et al. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health. 2017; 5:e665–72.4. Dewan P, Jhund PS, Shen L, et al. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia, Europe and the Americas. Eur J Heart Fail. 2019; 21:577–587.
Article5. Reyes EB, Ha JW, Firdaus I, et al. Heart failure across Asia: same healthcare burden but differences in organization of care. Int J Cardiol. 2016; 223:163–167.
Article6. Teng TK, Tromp J, Tay WT, et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. 2018; 6:e1008–18.
Article7. Hasan MS, Basri HB, Hin LP, Stanslas J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: why the Asian population requires special attention. Int J Neurosci. 2013; 123:143–154.
Article8. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371:993–1004.
Article9. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2017; 136:e137–e161.
Article10. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37:2129–2200.11. de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981; 28:89–94.
Article12. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007; 50:2357–2368.
Article13. O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011; 365:32–43.14. Packer M, O'Connor C, McMurray JJ, et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017; 376:1956–1964.
Article15. Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011; 278:1808–1817.
Article16. Kerr MA, Kenny AJ. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974; 137:477–488.
Article17. Bayes-Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016; 68:639–653.18. McKinnie SM, Fischer C, Tran KM, et al. The metalloprotease neprilysin degrades and inactivates apelin peptides. ChemBioChem. 2016; 17:1495–1498.
Article19. Roques BP, Fournié-Zaluski MC, Soroca E, et al. The enkephalinase inhibitor thiorphan shows antinociceptive activity in mice. Nature. 1980; 288:286–288.
Article20. Shepperson NB, Barclay PL, Bennett JA, Samuels GM. Inhibition of neutral endopeptidase (EC 3.4.24.11) leads to an atrial natriuretic factor-mediated natriuretic, diuretic and antihypertensive response in rodents. Clin Sci (Lond). 1991; 80:265–269.
Article21. Kahn JC, Patey M, Dubois-Rande JL, et al. Effect of sinorphan on plasma atrial natriuretic factor in congestive heart failure. Lancet. 1990; 335:118–119.
Article22. Gros C, Souque A, Schwartz JC, et al. Protection of atrial natriuretic factor against degradation: diuretic and natriuretic responses after in vivo inhibition of enkephalinase (EC 3.4.24.11) by acetorphan. Proc Natl Acad Sci U S A. 1989; 86:7580–7584.
Article23. Northridge DB, Jardine AG, Alabaster CT, et al. Effects of UK 69 578: a novel atriopeptidase inhibitor. Lancet. 1989; 2:591–593.
Article24. Northridge DB, Newby DE, Rooney E, Norrie J, Dargie HJ. Comparison of the short-term effects of candoxatril, an orally active neutral endopeptidase inhibitor, and frusemide in the treatment of patients with chronic heart failure. Am Heart J. 1999; 138:1149–1157.
Article25. Bevan EG, Connell JM, Doyle J, et al. Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension. J Hypertens. 1992; 10:607–613.26. Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998; 97:2323–2330.
Article27. Dalzell JR, Seed A, Berry C, et al. Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: studies with thiorphan and omapatrilat. Cardiovasc Ther. 2014; 32:13–18.
Article28. Robl JA, Sun CQ, Stevenson J, et al. Dual metalloprotease inhibitors: mercaptoacetyl-based fused heterocyclic dipeptide mimetics as inhibitors of angiotensin-converting enzyme and neutral endopeptidase. J Med Chem. 1997; 40:1570–1577.
Article29. Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000; 356:615–620.
Article30. Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation. 2002; 106:920–926.31. Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004; 17:103–111.
Article32. Fryer RM, Segreti J, Banfor PN, et al. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. 2008; 153:947–955.
Article33. Feng L, Karpinski PH, Sutton P, et al. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012; 53:275–276.
Article34. Ayalasomayajula S, Langenickel T, Pal P, Boggarapu S, Sunkara G. Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): a novel angiotensin receptor-neprilysin inhibitor. Clin Pharmacokinet. 2017; 56:1461–1478.
Article35. Cada DJ, Baker DE, Leonard J. Sacubitril/valsartan. Hosp Pharm. 2015; 50:1025–1036.
Article36. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001; 345:1667–1675.
Article37. Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010; 50:401–414.
Article38. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991; 325:293–302.
Article39. Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015; 36:1990–1997.
Article40. Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015; 36:2576–2584.
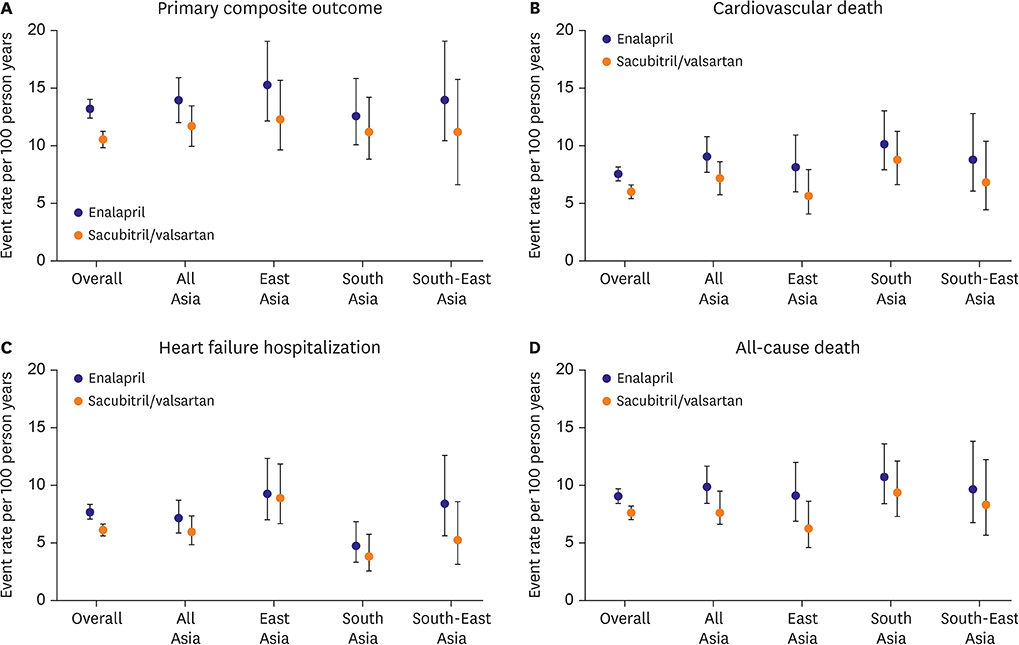
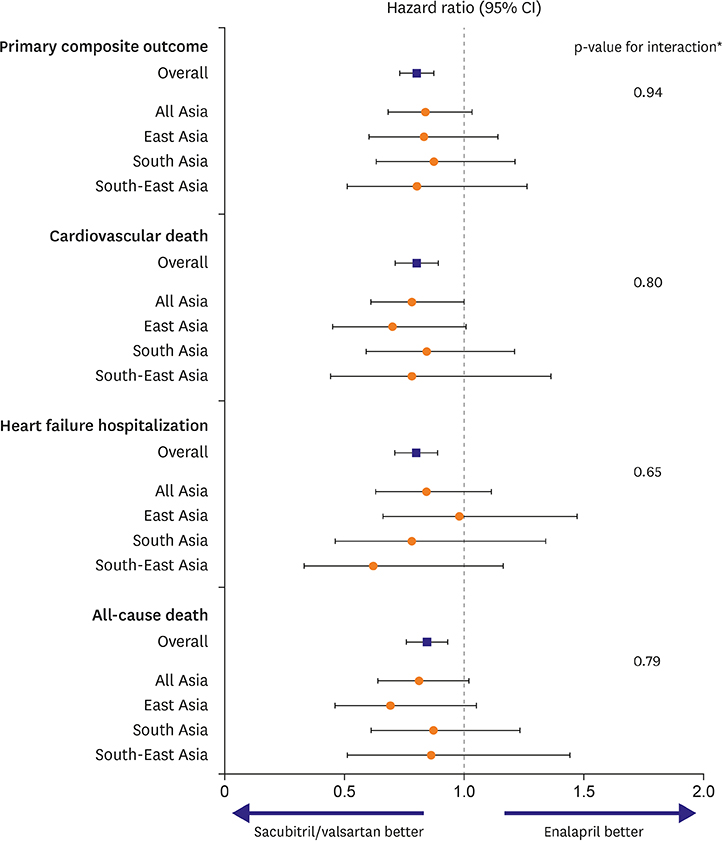
Article41. Kristensen SL, Martinez F, Jhund PS, et al. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J. 2016; 37:3167–3174.
Article42. Okumura N, Jhund PS, Gong J, et al. Effects of sacubitril/valsartan in the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Hear Fail. 2016; 9:e003212.
Article43. Cannon JA, Shen L, Jhund PS, et al. Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction. Eur J Heart Fail. 2017; 19:129–137.44. Pocock SJ, McMurray JJ, Collier TJ. Making sense of statistics in clinical trial reports: part 1 of a 4-part series on statistics for clinical trials. J Am Coll Cardiol. 2015; 66:2536–2549.45. Solomon SD, Claggett B, McMurray JJ, Hernandez AF, Fonarow GC. Combined neprilysin and renin-angiotensin system inhibition in heart failure with reduced ejection fraction: a meta-analysis. Eur J Heart Fail. 2016; 18:1238–1243.
Article46. Senni M, McMurray JJ, Wachter R, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016; 18:1193–1202.
Article47. Wachter R, Senni M, Belohlavek J, Butylin D, Noe A, Pascual-Figal D. Sacubitril/valsartan initiated in hospitalized patients with heart failure with reduced ejection fraction after hemodynamic stabilization: primary results of the TRANSITION study. J Card Fail. 2018; 24:S15.
Article48. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380:539–548.
Article49. Solomon SD, Rizkala AR, Gong J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail. 2017; 5:471–482.50. Solomon SD, Rizkala AR, Lefkowitz MP, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Hear Fail. 2018; 11:e004962.51. Tsutsui H, Momomura S, Saito Y, et al. Efficacy and safety of sacubitril/valsartan (LCZ696) in Japanese patients with chronic heart failure and reduced ejection fraction: rationale for and design of the randomized, double-blind PARALLEL-HF study. J Cardiol. 2017; 70:225–231.
Article52. Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995; 40:141–144.53. Mentz RJ, Roessig L, Greenberg BH, et al. Heart failure clinical trials in East and Southeast Asia: understanding the importance and defining the next steps. JACC Heart Fail. 2016; 4:419–427.54. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987; 316:1429–1435.55. Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992; 327:685–691.
Article56. Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991; 325:303–310.
Article57. Komajda M, Lutiger B, Madeira H, et al. Tolerability of carvedilol and ACE-inhibition in mild heart failure. Results of CARMEN (Carvedilol ACE-Inhibitor Remodelling Mild CHF EvaluatioN). Eur J Heart Fail. 2004; 6:467–475.
Article58. Willenheimer R, van Veldhuisen DJ, Silke B, et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005; 112:2426–2435.59. McMurray JJ, Krum H, Abraham WT, et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016; 374:1521–1532.
Article60. Heart Online Technology (CN). Guide: chronic HFrEF drug treatment, a summary! [Internet]. Beijing: Heart Online Technology;2018. cited 2019 Apr 10. Available from: http://www.heartonline.cn/themes/TPL_XZX003/views/details.html?id=5bd81316e12b371d4a5b8591&category=/information/infoSub&mark=/information/infoSub.61. Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017; 47:555–643.62. Wang CC, Chen JH, Yu WC, et al. 2012 Guidelines of the Taiwan Society of Cardiology (TSOC) for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2012; 28:161–195.63. Guha S, Harikrishnan S, Ray S, et al. CSI position statement on management of heart failure in India. Indian Heart J. 2018; 70:Suppl 1. S1–72.64. Ministry of Health Malaysia. Management of heart failure [Internet]. Putrajaya: Ministry of Health Malaysia;2014. cited 2019 Apr 10. Available from: http://www.moh.gov.my/moh/resources/Penerbitan/CPG/CARDIOVASCULAR/8.pdf.65. Buakhamsri A, Chirakarnjanakorn S, Sanguanwong S, Porapakkham P, Kanjanavanich R. Heart Failure Council of Thailand (HFCT) 2019 heart failure guideline: pharmacologic treatment of chronic heart failure - part I. J Med Assoc Thai. 2019; 102:240–244.66. Yingchoncharoen T, Kanjanavanich R. Heart Failure Council of Thailand (HFCT) 2019 heart failure guideline: pharmacologic treatment of chronic heart failure - part II. J Med Assoc Thai. 2019; 102:368–372.67. Ministry of Health, Singapore. Clinical practice guidelines: heart failure [Internet]. Singapore: Ministry of Health, Singapore;2004. cited 2019 Apr 10. Available from: https://www.moh.gov.sg/docs/librariesprovider4/guidelines/cpg_heart-failure-aug-2004.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac and kidney outcomes after sacubitril-valsartan therapy: recovery of cardiac function relative to kidney function decline
- ECG Monitoring of Reactions to Sacubitril-valsartan in Heart Failure with Reduced Ejection Fraction
- Angiotensin receptor-neprilysin inhibitor for the treatment of heart failure: a review of recent evidence
- Changes in QRS Duration Are Associated with a Therapeutic Response to Sacubitril–valsartan in Heart Failure with Reduced Ejection Fraction
- Expect the Unexpected in the Medical Treatment of Heart Failure with Reduced Ejection Fraction: between Scientific Evidence and Clinical Wisdom