J Korean Soc Radiol.
2019 May;80(3):466-476. 10.3348/jksr.2019.80.3.466.
Morphologic Evaluation of Primary Non-Small Cell Lung Cancer by 3 Tesla MRI with Free-Breathing Ultrashort Echo Time and Radial T1-Weighted Gradient Echo Sequences: A Comparison with CT Analysis
- Affiliations
-
- 1Department of Radiology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea. wckwon@yonsei.ac.kr
- 2Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
- 3Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2454026
- DOI: http://doi.org/10.3348/jksr.2019.80.3.466
Abstract
- PURPOSE
To evaluate morphologic features of primary non-small cell lung cancer using 3 Tesla MRI with free-breathing compared with CT.
MATERIALS AND METHODS
Thirty-six patients were enrolled. A 64-channel multidetector CT and 3 Tesla MRI with ultrashort echo time pointwise encoding time reduction with radial acquisition (PETRA) and radial volumetric interpolated breath-hold examination (VIBE) were compared in size, shape, margin, internal characteristics, and tumor interface of primary tumor.
RESULTS
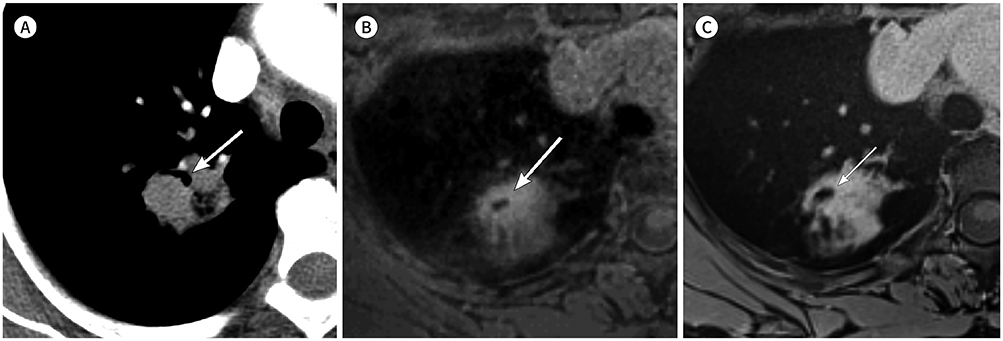
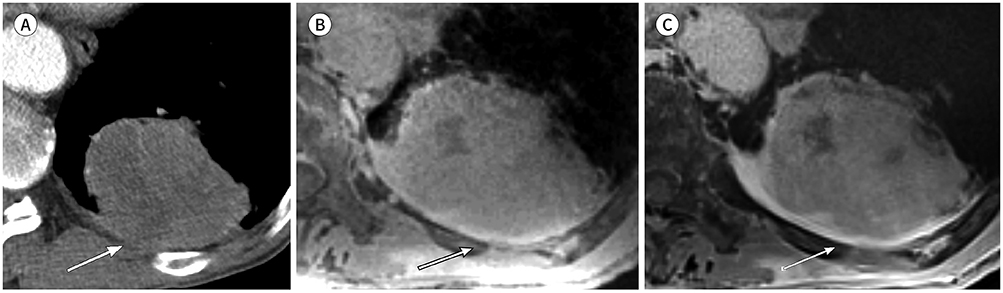
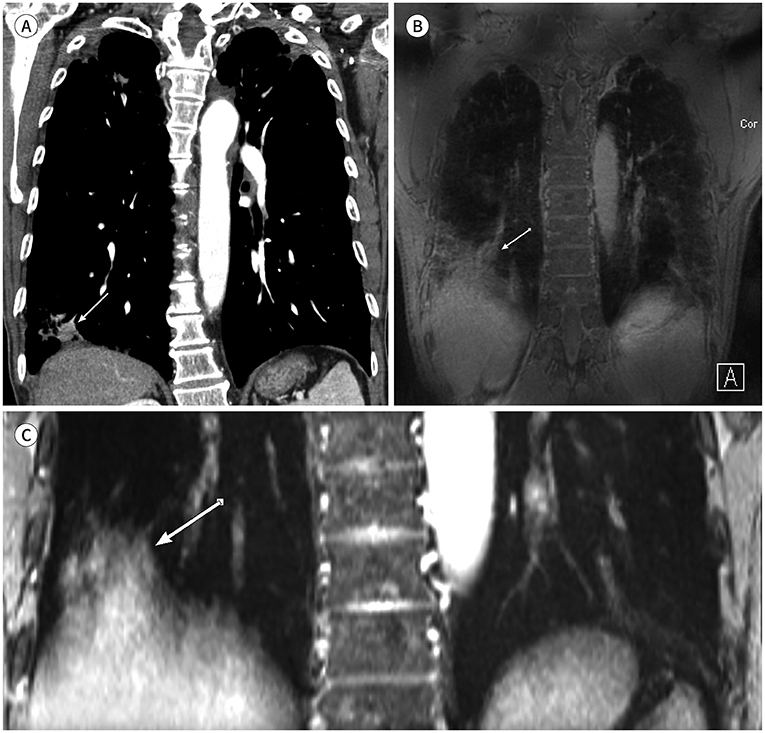
There were no significant differences in tumor size between CT and either PETRA or radial VIBE (p = 0.054 and p = 0.764, respectively). Kappa (κ) statistics of shape, margin, and internal characteristics were respectively κ = 0.86, 0.65, 0.77 on PETRA and κ = 0.93, 0.84, 0.83 on radial VIBE compared with CT. PETRA and radial VIBE revealed clearer interface compared with CT (p = 0.000 and p < 0.000, respectively). Radial VIBE showed higher frequency of clear interface (94.4%) than PETRA (88.9%). MRI did not show significantly clear interface which was located in lung base (p = 0.363 on PETRA and p = 0.175 on radial VIBE) compared with CT.
CONCLUSION
MRI with PETRA and radial VIBE sequences can be a feasible method to evaluate morphologic features of primary non-small cell lung cancer compared with CT.
MeSH Terms
Figure
Reference
-
1. Dournes G, Grodzki D, Macey J, Girodet PO, Fayon M, Chateil JF, et al. Quiet submillimeter MR imaging of the lung is feasible with a PETRA sequence at 1.5 T. Radiology. 2015; 276:258–226.
Article2. Kurihara Y, Matsuoka S, Yamashiro T, Fujikawa A, Matsushita S, Yagihashi K, et al. MRI of pulmonary nodules. AJR Am J Roentgenol. 2014; 202:W210–W216.
Article3. Stolzmann P, Veit-Haibach P, Chuck N, Rossi C, Frauenfelder T, Alkadhi H, et al. Detection rate, location, and size of pulmonary nodules in trimodality PET/CT-MR: comparison of low-dose CT and Dixon-based MR imaging. Invest Radiol. 2013; 48:241–246.4. Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med. 2012; 67:510–518.
Article5. Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013; 70:1241–1250.
Article6. Chandarana H, Heacock L, Rakheja R, DeMello LR, Bonavita J, Block TK, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology. 2013; 268:874–881.
Article7. Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol. 2011; 46:648–653.8. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer International Publishing;2017.9. Liu Y, Wang H, Li Q, McGettigan MJ, Balagurunathan Y, Garcia AL, et al. Radiologic features of small pulmonary nodules and lung cancer risk in the national lung screening trial: a nested case-control study. Radiology. 2018; 286:298–306.
Article10. Kuriyama K, Tateishi R, Doi O, Higashiyama M, Kodama K, Inoue E, et al. Prevalence of air bronchograms in small peripheral carcinomas of the lung on thin-section CT: comparison with benign tumors. AJR Am J Roentgenol. 1991; 156:921–924.
Article11. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003; 228:303–308.
Article12. Dournes G, Menut F, Macey J, Fayon M, Chateil JF, Salel M, et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol. 2016; 26:3811–3820.
Article13. Bannas P, Bell LC, Johnson KM, Schiebler ML, François CJ, Motosugi U, et al. Pulmonary embolism detection with three-dimensional ultrashort echo time MR imaging: experimental study in canines. Radiology. 2016; 278:413–421.
Article14. Biederer J, Mirsadraee S, Beer M, Molinari F, Hintze C, Bauman G, et al. MRI of the lung (3/3)-current applications and future perspectives. Insights Imaging. 2012; 3:373–386.
Article15. Purandare NC, Rangarajan V. Imaging of lung cancer: implications on staging and management. Indian J Radiol Imaging. 2015; 25:109–120.
Article16. Gai ND, Malayeri A, Agarwal H, Evers R, Bluemke D. Evaluation of optimized breath-hold and free-breathing 3D ultrashort echo time contrast agent-free MRI of the human lung. J Magn Reson Imaging. 2016; 43:1230–1238.
Article17. Landwehr P, Schulte O, Lackner K. MR imaging of the chest: mediastinum and chest wall. Eur Radiol. 1999; 9:1737–1744.
Article18. Schwenzer NF, Seith F, Gatidis S, Brendle C, Schmidt H, Pfannenberg CA, et al. Diagnosing lung nodules on oncologic MR/PET imaging: comparison of fast T1-weighted sequences and influence of image acquisition in inspiration and expiration breath-hold. Korean J Radiol. 2016; 17:684–694.
Article19. Semelka RC, Cem Balci N, Wilber KP, Fisher LL, Brown MA, Gomez-Caminero A, et al. Breath-hold 3D gradient-echo MR imaging of the lung parenchyma: evaluation of reproducibility of image quality in normals and preliminary observations in patients with disease. J Magn Reson Imaging. 2000; 11:195–200.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imaging Assessment of Visceral Pleural Surface Invasion by Lung Cancer: Comparison of CT and Contrast-Enhanced Radial T1-Weighted Gradient Echo 3-Tesla MRI
- T1-weighted MR Imaging of the Neonatal Brain at 3.0 Tesla: Comparison of Spin Echo, Fast Inversion Recovery, and Magnetization-prepared Three Dimensional Gradient Echo Techniques
- Ultrashort Echo Time and Zero Echo Time MRI and Their Applications at High Magnetic Fields: A Literature Survey
- Gradient Optimized Gradient-Echo Gradient Moment Nulling Sequences for Flow Compensation of Brain mages
- An exeprimental study on MRI imaging of jugular venous thrombosis in dogs