Anat Cell Biol.
2019 Jun;52(2):204-207. 10.5115/acb.2019.52.2.204.
Chondroid metaplasia of paraspinal connective tissue in the degenerative spine
- Affiliations
-
- 1Hasselt University, Rehabilitation Research Center, BIOMED Biomedical Research Institute, Faculty of Medicine and Life Sciences, Diepenbeek, Belgium. sjoerd.stevens@uhasselt.be
- 2Department of Pathology, Jessa Hospital, Hasselt, Belgium.
- 3Department of Veterinary Medicine, Integrated Veterinary Research Unit-Namur Research Institute for Life Science (IVRU-NARILIS), Faculty of Sciences, University of Namur, Namur, Belgium.
- 4Department of Morphology, Hasselt University, Faculty of Medicine and Life Sciences, Diepenbeek, Belgium.
- KMID: 2451225
- DOI: http://doi.org/10.5115/acb.2019.52.2.204
Abstract
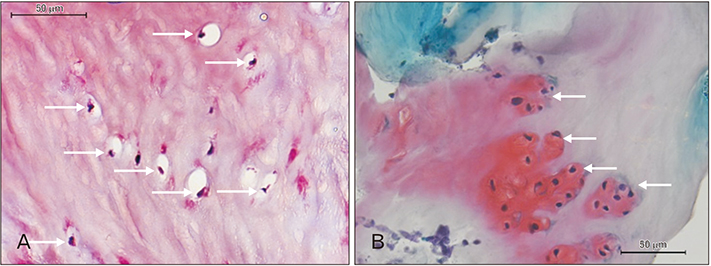
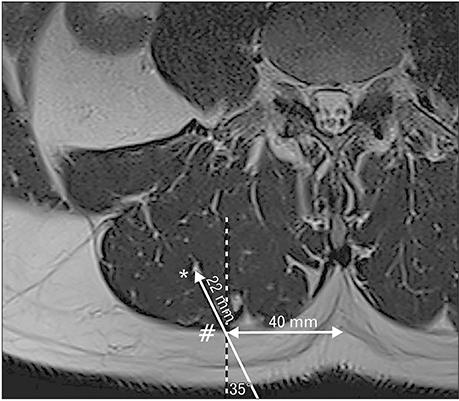
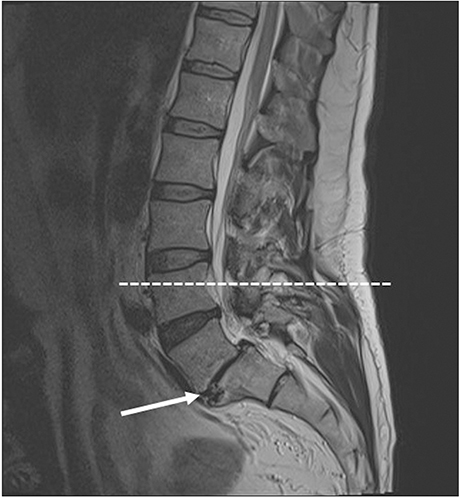
- A 51-year-old male was routinely biopsied during a paraspinal muscle study. The biopsy sample was taken from the right erector spinae muscle at the fourth lumbar vertebra. The patient had no history of (diagnosed) major back trauma. The obtained sample was histologically analyzed (hematoxylin and eosin, safranin O), and complementary magnetic resonance imaging was performed. The biopsied sample contained chondroid tissue. Based on its location, the biopsy sample was appointed as chondroid metaplasia. Although chondroid metaplasia is not uncommon in humans, this is the first report of chondroid metaplasia within the paraspinal connective tissue. We propose a novel mechanism to explain the paraspinal chrondrogenic changes, related to spinal degeneration.
MeSH Terms
Figure
Reference
-
1. Bogduk N. A reappraisal of the anatomy of the human lumbar erector spinae. J Anat. 1980; 131:525–540.2. Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012; 221:507–536.
Article3. Clemente CD. Gray's anatomy of the human body. Philadelphia, PA: Lea & Febiger;1985.4. Zullo A, Mancini FP, Schleip R, Wearing S, Yahia L, Klingler W. The interplay between fascia, skeletal muscle, nerves, adipose tissue, inflammation and mechanical stress in musculo-fascial regeneration. J Gerontol Geriatr. 2017; 65:271–283.5. Uezumi A, Ikemoto-Uezumi M, Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol. 2014; 5:68.
Article6. Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012; 27:1004–1017.
Article7. Shon W, Folpe AL. Myxochondroid metaplasia of the plantar foot: a distinctive pseudoneoplastic lesion resembling nuchal fibrocartilaginous pseudotumor and the equine digital cushion. Mod Pathol. 2013; 26:1561–1567.
Article8. Myint ZW, Raparla S, Kamugisha LK. Metaplastic breast cancer with chondroid differentiation. J Community Hosp Intern Med Perspect. 2015; 5:28935.
Article9. Agten A, Verbrugghe J, Stevens S, Boomgaert L, B OE, Timmermans A, Vandenabeele F. Feasibility, accuracy and safety of a percutaneous fine-needle biopsy technique to obtain qualitative muscle samples of the lumbar multifidus and erector spinae muscle in persons with low back pain. J Anat. 2018; 233:542–551.
Article10. Thakar S, Sivaraju L, Aryan S, Mohan D, Sai Kiran NA, Hegde AS. Lumbar paraspinal muscle morphometry and its correlations with demographic and radiological factors in adult isthmic spondylolisthesis: a retrospective review of 120 surgically managed cases. J Neurosurg Spine. 2016; 24:679–685.
Article11. Park MS, Moon SH, Kim TH, Oh J, Lee SJ, Chang HG, Shin JH. Paraspinal muscles of patients with lumbar diseases. J Neurol Surg A Cent Eur Neurosurg. 2018; 79:323–329.
Article12. Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA, Devarakonda PM, Schneider MJ Jr, Cummins SM, Legendre NP, Yamamoto S, Kaartinen V, Hunter JW, Goldhamer DJ. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018; 9:471.
Article13. James G, Sluka KA, Blomster L, Hall L, Schmid AB, Shu CC, Little CB, Melrose J, Hodges PW. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur Spine J. 2018; 27:1744–1756.
Article14. Hodges P, James G, Blomster L, Hall L, Schmid A, Shu C, et al. Multifidus muscle undergoes structural remodeling of muscle, adipose and connective tissue, but not atrophy after injury: molecular and morphological evidence. Physiotherapy. 2015; 101:Suppl 1. e581.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metaplastic Carcinoma of the Breast with Chondroid Calcification: A Case Report
- Postoperative Chondroid Metaplasia in First Metacarpocarpal Joint: A Case Report
- Endometrial Osseous Metaplasia: Sonographic Findings
- Morphological Changes in the Ligamentum Flavum in Degenerative Lumbar Canal Stenosis: A Prospective, Comparative Study
- Tissue expansion for the paraspinal soft tissue reconstruction in lumbar spine fracture-dislocation: a case report